

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
General trends
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
الجزء والصفحة:
ص593-594
2025-10-07
152
General trends
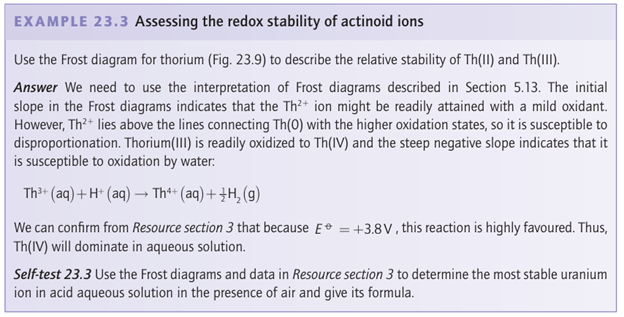
Key point: The early actinoids do not exhibit the chemical uniformity of the lanthanoids; they exist in diverse oxidation states with An (III) progressively more stable across the series. The 14 elements from thorium (Th, Z=90, 5f1) to lawrencium (Lr, Z=103, 5f14) involve the progressive completion of the 5f subshell, and in this sense are analogues of the lanthanoids. However, the actinoids do not exhibit the chemical uniformity of the lanthanoids. Whereas, like the lanthanoids, a common oxidation state of the actinoids is An (III), unlike the lanthanoids the early members of the series occur in a rich variety of other oxidation states. The Frost diagrams in Fig. 23.9 show that oxidation numbers higher than 3 are easily accessible and often preferred for the early elements of the block (Th, Pa, U, Np, Pu) whereas 3 becomes predominant in Am and beyond. Linear or nearly linear AnO2 and AnO2+2 ions are the dominant aqua species for oxidation numbers +5 and +6 exhibited by U and Np. Unlike in the lanthanoids, the f orbitals of the early actinoids extend into the bonding region, so the spectra of their complexes are strongly affected by ligands. The 5f and 6d orbitals are less compact than the 4f and 5d orbitals and the electrons in them are more available for bonding. Thus, the outermost electron configuration of U is 5f36d17s2 with all six electrons available for bonding. At around Am and Cm the 5f and 6d orbital energies are lower due to their poor screening by the other electrons, so that the later actinoids behave like the lanthanoids with only the outermost three electrons being readily ionized.
The striking differences between the chemical properties of the lanthanoids and early actinoids led to controversy about the most appropriate placement of the actinoids in the periodic table. For example, before 1945, periodic tables usually showed U below W because both elements have a maximum oxidation number of +6. The emergence of oxidation states An (III) for the later actinoids was a key point in determining their current placement. The similarity of the heavy actinoids and the lanthanoids is illustrated by their similar elution behaviour in ion-exchange separation (compare Figs 23.8 and 23.10).
Like the lanthanoids, the actinoids have large atomic and ionic radii (the radius of an An3+ ion is typically about 5 pm larger than its Ln3 congener) and as a result often have high coordination numbers. For example, uranium in solid UCl4 is eight-coordinate and in solid UBr4 it is seven-coordinate in a pentagonal-bipyramidal array. Solid-state structures with coordination numbers up to 12 have been observed. Because of the small quantities of material available in most cases and their intense radioactivity, most of the chemical properties of the trans americium elements (the elements following americium, Z=95) have been established by experiments carried out on a microgram scale or even on just a few hundred atoms. For example, the trans americium ion complexes have been adsorbed on and eluted from a single bead of ion-exchange material of diameter 0.2 mm. For the heaviest and most unstable post-actinoids, such as hassium (Hs, Z=108), the lifetimes are too short for chemical separation and the identification of the element is based exclusively on the properties of the radiation it emits.
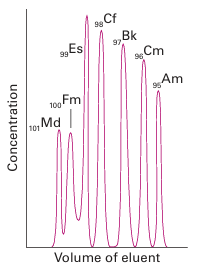
Figure 23.10 Elution of heavy actinoid ions from a cation exchange column using ammonium 2-hydroxyisobutyrate as the eluent. Note the similarity in elution sequence to Fig. 23.8: the heavy (smaller) An3 ions elute first.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















