

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Biomineralization
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
الجزء والصفحة:
ص771-772
2025-10-28
1078
Biomineralization
Key points: Calcium compounds are used in exoskeletons, bones, teeth, and other devices; some organ isms use crystals of magnetite, Fe3O4, as a compass; plants produce silica-based protective devices. Biominerals can be either infinite covalent networks or ionic. The former include the silicates, which occur extensively in the plant world. Leaves, even whole plants, are often covered with silica hairs or spines that offer protection against predatory herbivores. Ionic biominerals are mainly based on calcium salts, and exploit the high lattice energy and low solubilities of these compounds. Calcium carbonate (calcite or aragonite, Section 12.10) is the material present in sea shells and eggshells. These minerals persist long after the organism has died, indeed chalk is a biogenic mineral, a result of the process of calciferation of prehistoric organisms. Calcium phosphate (hydroxyapatite, Section 15.4) is the mineral component of bones and teeth, which are particularly good examples of how organisms fabricate ‘living’ composite materials. Indeed, the different properties of bone found among species (such as stiffness) are produced by varying the amount of organic component, mostly the fibrous protein collagen, with which hydroxyapatite is associated. High hydroxyapatite/collagen ratios are found for large marine animals, whereas low ra tios are found for animals requiring agility and elasticity. Biomineralization is crystallization under biological control. There are some striking examples that have no counterparts in the laboratory. Perhaps the most familiar example is the exoskeleton of the sea urchin, which comprises large sponge-like plates containing continuous macropores 15 µm in diameter: each of these plates is a single crystal of Mg rich calcite. Large single crystals support other organisms, such as diatoms and radiolarians, with their skeletons of silica cages (Fig. 27.54). Biominerals produce some intriguing gadgetry. Figure 27.55 shows crystals of calcite that are part of the gravity sensor device in the inner ear. These crystals are located on a membrane above sensory cells and any acceleration or change of posture that causes them to move results in an electrical signal to the brain. The crystals are uniform in size and spindle-shaped so that they move evenly without becoming hooked together. The same property is seen for crystals of magnetite (Fe3O4) that are found in a variety of magnetotactic bacteria (Fig. 27.56). There are considerable variations in size and shape across different species, but within any one species, the crystals, formed in magnetosome vesicles, are uniform. Magnetotactic bacteria live in fluid sediment suspensions in marine and freshwater environments, and it is thought that their microcompasses allow them to swim always in a downwards direction to maintain their chemical environment during turbulent conditions. There is great interest in how biomaterials are formed, not least because of the in spiration they provide for nanotechnology (Chapter 25). The formation of biominerals involves the following hierarchy of control mechanisms:
1. Chemical control (solubility, supersaturation, nucleation). 2. Spatial control (confinement of crystal growth by boundaries such as cells, subcompartments, and even proteins in the case of ferritin, Section 27. 6). 3. Structural control (nucleation is favoured on a specific crystal face). 4. Morphological control (growth of the crystal is limited by boundaries imposed by organic material that grows with time). 5. Constructional control (interweaving inorganic and organic materials to form a higher order structure, such as bone). Bone is continually being dissolved and reformed; indeed it functions not only as a structural support but also as the central Ca store. Thus, during pregnancy, bones tend to be raided for their Ca in a process called demineralization, which occurs in special cells called osteoclasts. Depleted or damaged bones are restored by mineralization, which occurs in cells called osteoblasts. These processes involve phosphatases (Section 27.9).
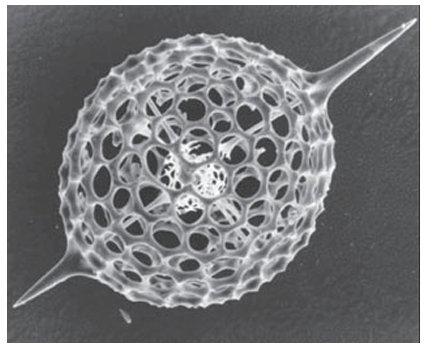
Figure 27.54 The porous silica structure of a radiolarian microskeleton showing the large radial spines. (Photograph supplied by Professor S. Mann, University of Bristol.)
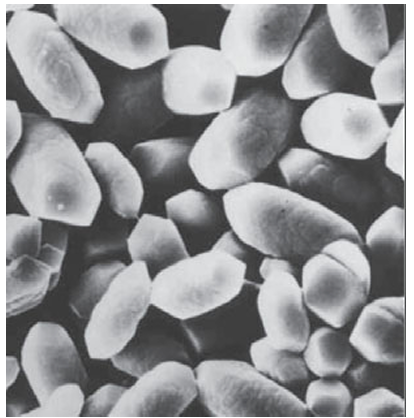
Figure 27.55 Our gravity sensor. Crystals of biologically formed calcite that are found in the inner ear. (Photograph supplied by Professor S. Mann, University of Bristol.)
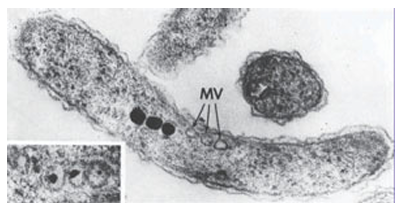
Figure 27.56 Magnetite crystals in magnetotactic bacteria. These are tiny compasses that guide these organisms to move vertically in river bed sludge. MV indicates empty vesicles (Photograph supplied by Professor S. Mann, University of Bristol.)
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















