
آخر المواضيع المضافة


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 5-4-2017
Date: 14-5-2020
Date: 10-5-2020
|
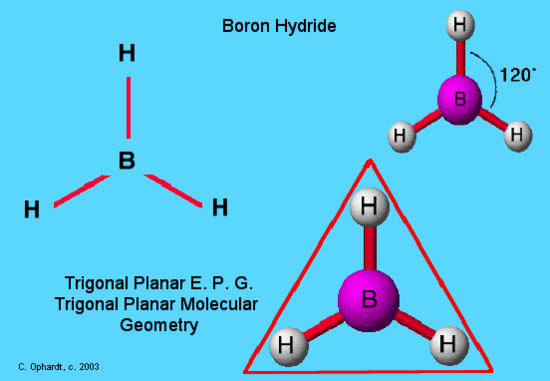
An example of trigonal planar electron pair geometry and molecular geometry is BH3. This molecule is electron deficient and does not follow the octet rule because it has only 6 valence electrons. The hydrogen atoms are as far apart as possible at 120o. This is trigonal planar geometry. The molecule all in a plane and is two dimensional.This molecule exists in a gaseous state in only minute quantities under specialized conditions as an intermediate in the making of other boron hydride type molecules.



|
|
|
|
دراسة: حفنة من الجوز يوميا تحميك من سرطان القولون
|
|
|
|
|
|
|
تنشيط أول مفاعل ملح منصهر يستعمل الثوريوم في العالم.. سباق "الأرنب والسلحفاة"
|
|
|
|
|
|
|
العتبة العباسية المقدسة تكرّم الفائزين بمسابقة فنِّ الخطابة الثامنة
|
|
|