
آخر المواضيع المضافة


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 14-11-2019
Date: 12-7-2018
Date: 5-8-2018
|
The mechanism is catalyzed by the addition of an acid or base. Note! This may speed up the reaction but is has not effect on the equilibriums discussed above. Basic conditions speed up the reaction because hydroxide is a better nucleophilic than water. Acidic conditions speed up the reaction because the protonated carbonyl is more electrophilic.
1) Nucleophilic attack by hydroxide
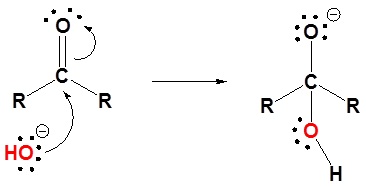
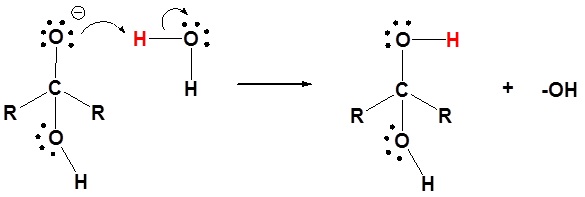
2) Protonation of the alkoxide
1) Protonation of the carbonyl

2) Nucleophilic attack by water
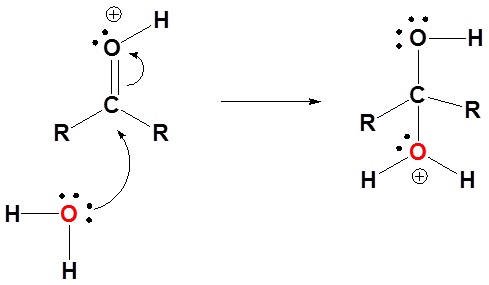
3) Deprotonation
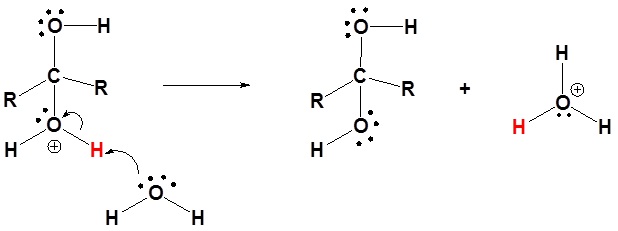



|
|
|
|
دراسة: حفنة من الجوز يوميا تحميك من سرطان القولون
|
|
|
|
|
|
|
تنشيط أول مفاعل ملح منصهر يستعمل الثوريوم في العالم.. سباق "الأرنب والسلحفاة"
|
|
|
|
|
|
|
لتعزيز التواصل مع الزائرات الأجنبيات : العتبة العلويّة المقدّسة تُطلق دورة لتعليم اللغة الإنجليزية لخادمات القسم النسويّ
|
|
|