


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 2-1-2020
Date: 13-8-2018
Date: 10-8-2019
|
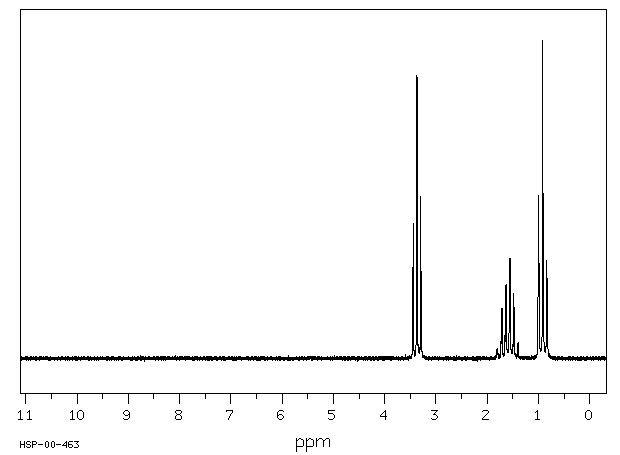
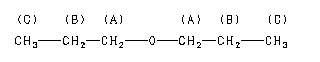
The 1H NMR spectrum of dipropyl ether shows three signals with the triplet at 3.37 ppm assigned to the -CH2- beside the ether and the other two signals upfield (1.59 and 0.93 ppm). Notice the protons closer to the electron withdrawing oxygen atom are further downfield indicating some deshielding. Protons at (A) and (C) are each coupled to two equivalent (B) protons. So, each of these signals appears as a triplet. The (B) protons in turn are coupled to a set of two and three equivalent protons and you would therefore formally expect a quartet of triplets. However, because the coupling constants are very similar, the signal appears as a sextet. Source: SDBSWeb : http://sdbs.db.aist.go.jp (National Institute of Advanced Industrial Science and Technology, 28 June 2017)



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
ضمن مؤتمر ذاكرة الألم في العراق مدير كرسي اليونسكو في جامعة الموصل يقدّم دراسةً تناقش استراتيجية الكرسي لنبذ التطرف وتعزيز ثقافة السلام
|
|
|