
آخر المواضيع المضافة


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 28-2-2017
Date: 17-11-2020
Date: 12-11-2020
|
Cesium Fluoride, CsF - mp/bp 703/1231 °C, (8,8) coordination.
With five shells of electrons shielding its nucleus, the Cs+ ion with its low charge density resembles a big puff-ball which can be distorted by the highly polarizing fluoride ion. The resulting ion-induced dipoles (blue arrows) account for much of the lattice energy here.
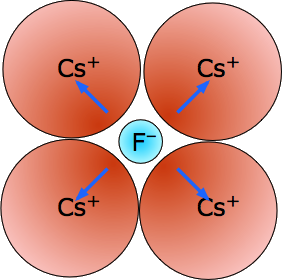
The reverse of this would be a tiny metal ion trying to hold onto four relatively huge iodide ions like Lithium iodide.
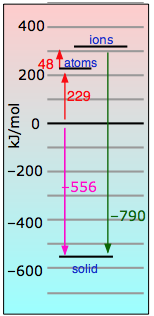



|
|
|
|
دراسة: حفنة من الجوز يوميا تحميك من سرطان القولون
|
|
|
|
|
|
|
تنشيط أول مفاعل ملح منصهر يستعمل الثوريوم في العالم.. سباق "الأرنب والسلحفاة"
|
|
|
|
|
|
|
لتعزيز التواصل مع الزائرات الأجنبيات : العتبة العلويّة المقدّسة تُطلق دورة لتعليم اللغة الإنجليزية لخادمات القسم النسويّ
|
|
|