


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 25-11-2021
Date: 28-12-2021
Date: 4-12-2021
|
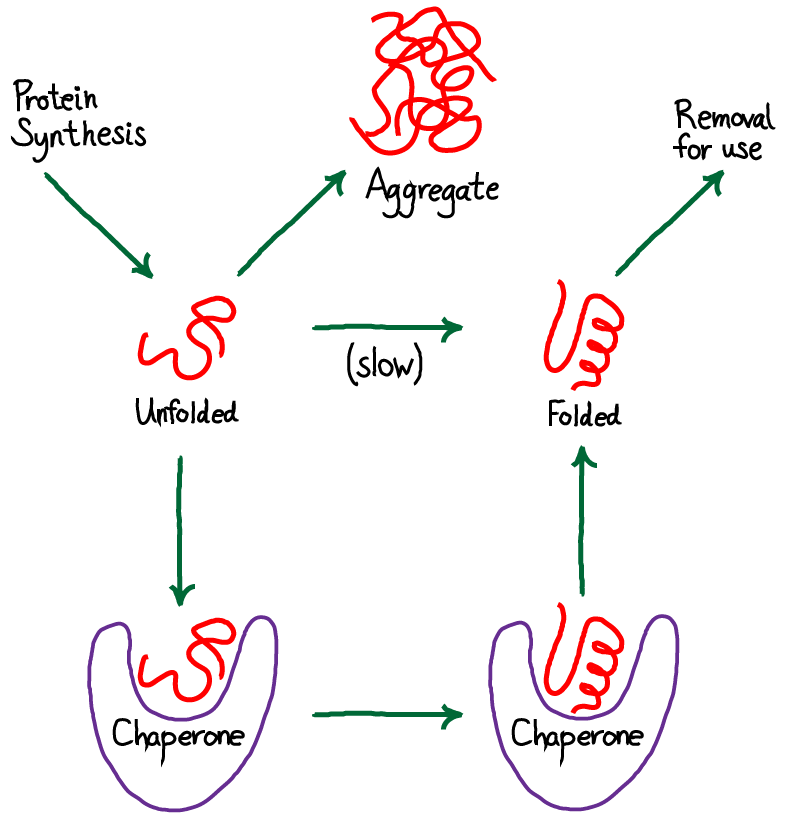
Chaperones in protein folding
The information needed for correct protein folding is contained in the primary structure of the polypeptide. However, most denatured proteins do not resume their native conformations even under favorable environmental conditions. This is because, for many proteins, folding is a facilitated process that requires a specialized group of proteins, referred to as molecular chaperones, and ATP hydrolysis. The chaperones, also known as heat shock proteins (HSP), interact with a polypeptide at various stages during the folding process. Some chaperones bind hydrophobic regions of an extended polypeptide and are important in keeping the protein unfolded until its synthesis is completed (for example, Hsp70). Others form cage-like macromolecular structures composed of two stacked rings. The partially folded protein enters the cage, binds the central cavity through hydrophobic interactions, folds, and is released (for example, mitochondrial Hsp60).[Note: Cage-like chaperones are sometimes referred to as chaperonins.] Chaperones, then, facilitate correct protein folding by binding to and stabilizing exposed, aggregation-prone hydrophobic regions in nascent (and denatured) polypeptides, preventing premature folding.



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
دراسة تستعرض آلام السجناء السياسيين في حقبة البعث المجرم في العراق
|
|
|