

النبات

مواضيع عامة في علم النبات

الجذور - السيقان - الأوراق

النباتات الوعائية واللاوعائية

البذور (مغطاة البذور - عاريات البذور)

الطحالب

النباتات الطبية


الحيوان

مواضيع عامة في علم الحيوان

علم التشريح

التنوع الإحيائي

البايلوجيا الخلوية


الأحياء المجهرية

البكتيريا

الفطريات

الطفيليات

الفايروسات


علم الأمراض

الاورام

الامراض الوراثية

الامراض المناعية

الامراض المدارية

اضطرابات الدورة الدموية

مواضيع عامة في علم الامراض

الحشرات


التقانة الإحيائية

مواضيع عامة في التقانة الإحيائية


التقنية الحيوية المكروبية

التقنية الحيوية والميكروبات

الفعاليات الحيوية

وراثة الاحياء المجهرية

تصنيف الاحياء المجهرية

الاحياء المجهرية في الطبيعة

أيض الاجهاد

التقنية الحيوية والبيئة

التقنية الحيوية والطب

التقنية الحيوية والزراعة

التقنية الحيوية والصناعة

التقنية الحيوية والطاقة

البحار والطحالب الصغيرة

عزل البروتين

هندسة الجينات


التقنية الحياتية النانوية

مفاهيم التقنية الحيوية النانوية

التراكيب النانوية والمجاهر المستخدمة في رؤيتها

تصنيع وتخليق المواد النانوية

تطبيقات التقنية النانوية والحيوية النانوية

الرقائق والمتحسسات الحيوية

المصفوفات المجهرية وحاسوب الدنا

اللقاحات

البيئة والتلوث


علم الأجنة

اعضاء التكاثر وتشكل الاعراس

الاخصاب

التشطر

العصيبة وتشكل الجسيدات

تشكل اللواحق الجنينية

تكون المعيدة وظهور الطبقات الجنينية

مقدمة لعلم الاجنة


الأحياء الجزيئي

مواضيع عامة في الاحياء الجزيئي


علم وظائف الأعضاء


الغدد

مواضيع عامة في الغدد

الغدد الصم و هرموناتها

الجسم تحت السريري

الغدة النخامية

الغدة الكظرية

الغدة التناسلية

الغدة الدرقية والجار الدرقية

الغدة البنكرياسية

الغدة الصنوبرية

مواضيع عامة في علم وظائف الاعضاء

الخلية الحيوانية

الجهاز العصبي

أعضاء الحس

الجهاز العضلي

السوائل الجسمية

الجهاز الدوري والليمف

الجهاز التنفسي

الجهاز الهضمي

الجهاز البولي


المضادات الميكروبية

مواضيع عامة في المضادات الميكروبية

مضادات البكتيريا

مضادات الفطريات

مضادات الطفيليات

مضادات الفايروسات

علم الخلية

الوراثة

الأحياء العامة

المناعة

التحليلات المرضية

الكيمياء الحيوية

مواضيع متنوعة أخرى

الانزيمات
Pyruvate Synthesis and ATP Production
المؤلف:
Denise R. Ferrier
المصدر:
Lippincott Illustrated Reviews: Biochemistry
الجزء والصفحة:
17-9-2021
2359
Pyruvate Synthesis and ATP Production
The conversion of PEP to pyruvate, catalyzed by pyruvate kinase (PK), is the third irreversible reaction of glycolysis. The high-energy enol phosphate in PEP is used to synthesize ATP from ADP and is another example of substrate-level phosphorylation .
1. Feedforward regulation: PK is activated by fructose 1,6-bisphosphate, the product of the PFK-1 reaction. This feedforward (instead of the more usual feedback) regulation has the effect of linking the two kinase activities: increased PFK-1 activity results in elevated levels of fructose 1,6-bisphosphate, which activates PK. [Note: PK is inhibited by ATP.]
2. Covalent regulation in the liver: Phosphorylation by cAMP-dependent PKA leads to inactivation of the hepatic isozyme of PK (Fig. 1).
When blood glucose levels are low, elevated glucagon increases the intracellular level of cAMP, which causes the phosphorylation and inactivation of PK in the liver only. Therefore, PEP is unable to continue in glycolysis and, instead, enters the gluconeogenesis pathway. This partly explains the observed inhibition of hepatic glycolysis and stimulation of gluconeogenesis by glucagon. Dephosphorylation of PK by a phosphatase results in reactivation of the enzyme.
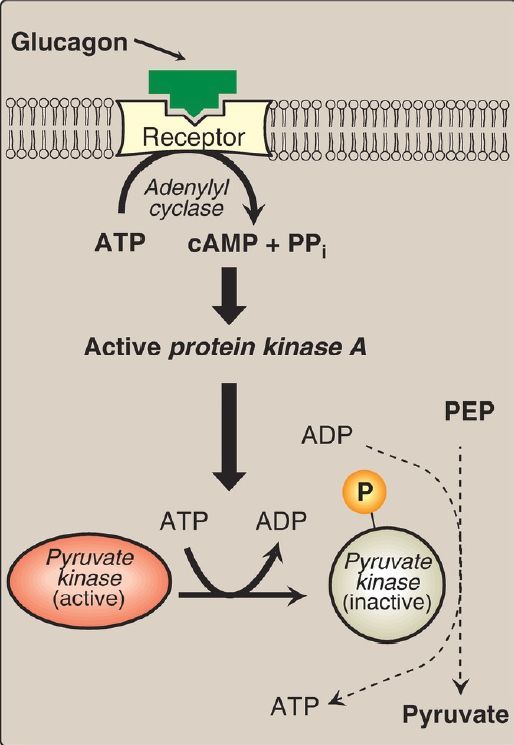
Figure 1: Covalent modification of hepatic pyruvate kinase results in inactivation of the enzyme. cAMP = cyclic adenosine monophosphate; PEP = phosphoenolpyruvate; = phosphate; PPi = pyrophosphate; ADP = adenosine diphosphate.
3. Pyruvate kinase deficiency: Because mature RBC lack mitochondria, they are completely dependent on glycolysis for ATP production. ATP is required to meet the metabolic needs of RBC and to fuel the ion pumps necessary for the maintenance of the flexible, biconcave shape that allows them to squeeze through narrow capillaries. The anemia observed in glycolytic enzyme deficiencies is a consequence of the reduced rate of glycolysis, leading to decreased ATP production by substrate-level phosphorylation. The resulting alterations in the RBC membrane lead to changes in cell shape and, ultimately, to phagocytosis by cells of the mononuclear phagocyte system, particularly splenic macrophages. The premature death and lysis of RBC result in mild-to-severe nonspherocytic hemolytic anemia, with the severe form requiring regular transfusions. Among patients with rare genetic defects of glycolytic enzymes, the majority has a deficiency in PK. [Note: Liver PK is encoded by the same gene as the RBC isozyme. However, liver cells show no effect because they can synthesize more PK and can also generate ATP by oxidative phosphorylation.] Severity depends both on the degree of enzyme deficiency (generally 5%–35% of normal levels) and on the extent to which RBC compensate by synthesizing increased levels of 2,3-BPG (. Almost all individuals with PK deficiency have a mutant enzyme that shows altered kinetics or decreased stability (Fig. 2). Individuals heterozygous for PK deficiency have resistance to the most severe forms of malaria.
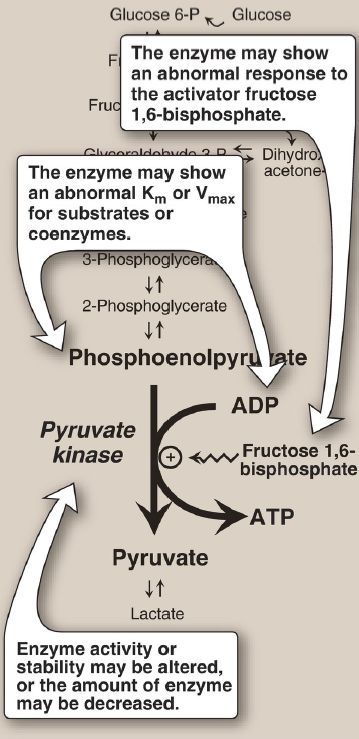
Figure 2: Alterations observed with various mutant forms of pyruvate kinase. Km = Michaelis constant; Vmax = maximal velocity; ADP = adenosine diphosphate.
The tissue-specific expression of PK in RBC and the liver results from the use of different start sites in transcription of the gene that encodes the enzyme.
 الاكثر قراءة في الكيمياء الحيوية
الاكثر قراءة في الكيمياء الحيوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















