


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 19-6-2019
Date: 19-8-2016
Date: 23-6-2019
|
Bonding in d-block metal complexes: valence bond theory
Hybridization schemes
Although VB theory in the form developed by Pauling in the 1930s is not much used now in discussing d-block metal complexes, the terminology and many of the ideas have been retained and some knowledge of the theory remains essential.
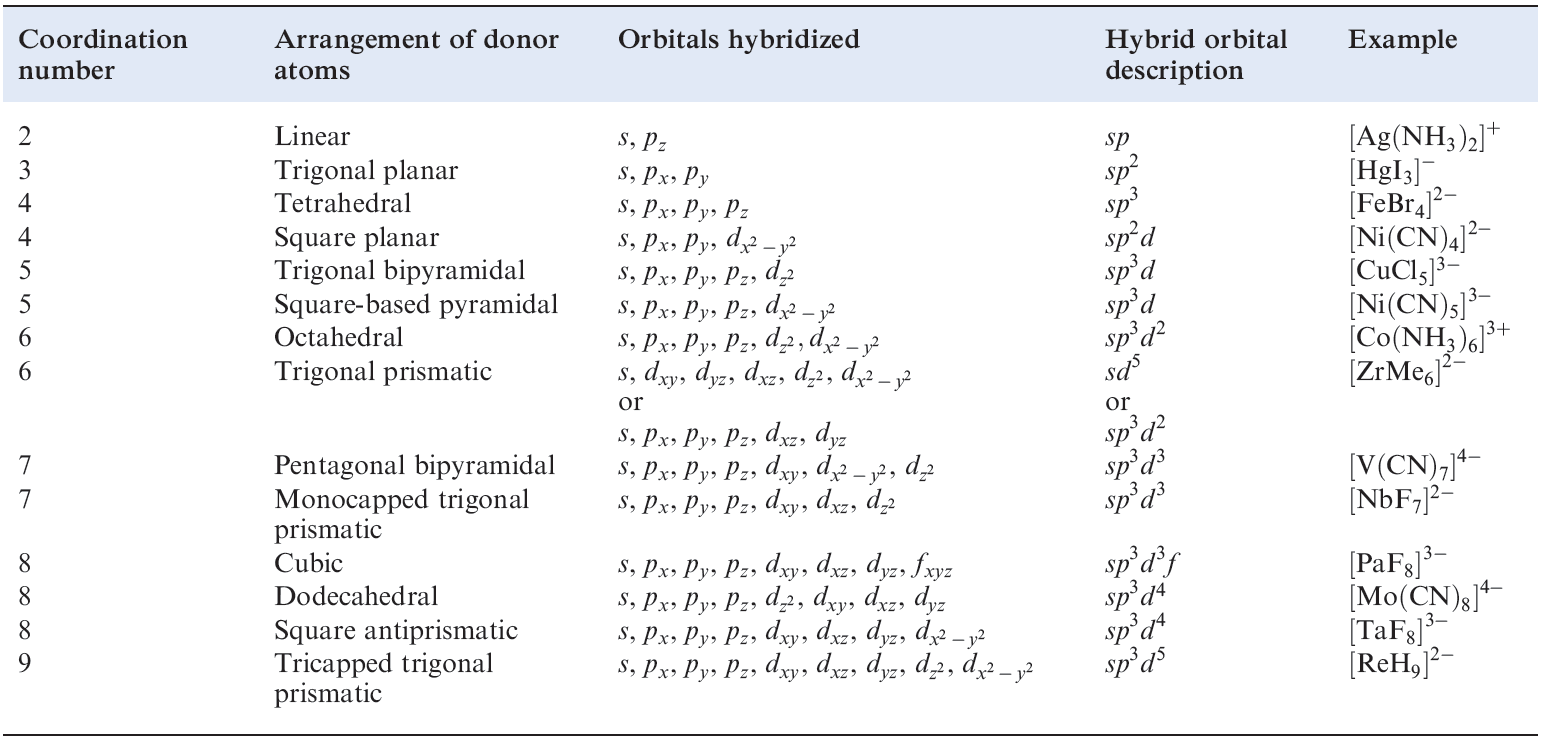
These same hybridization schemes can be used to describe the bonding in d-block metal complexes (Table 1.1); an empty hybrid orbital on the metal centre can accept a pair of electrons from a ligand to form a σ-bond. The choice of particular p or d atomic orbitals may depend on the definition of the axes with respect to the molecular framework, e.g. in linear ML2, the M–L vectors are defined to lie along the z axis. We have included the cube in Table 1.1 only to point out the required use of an f orbital.
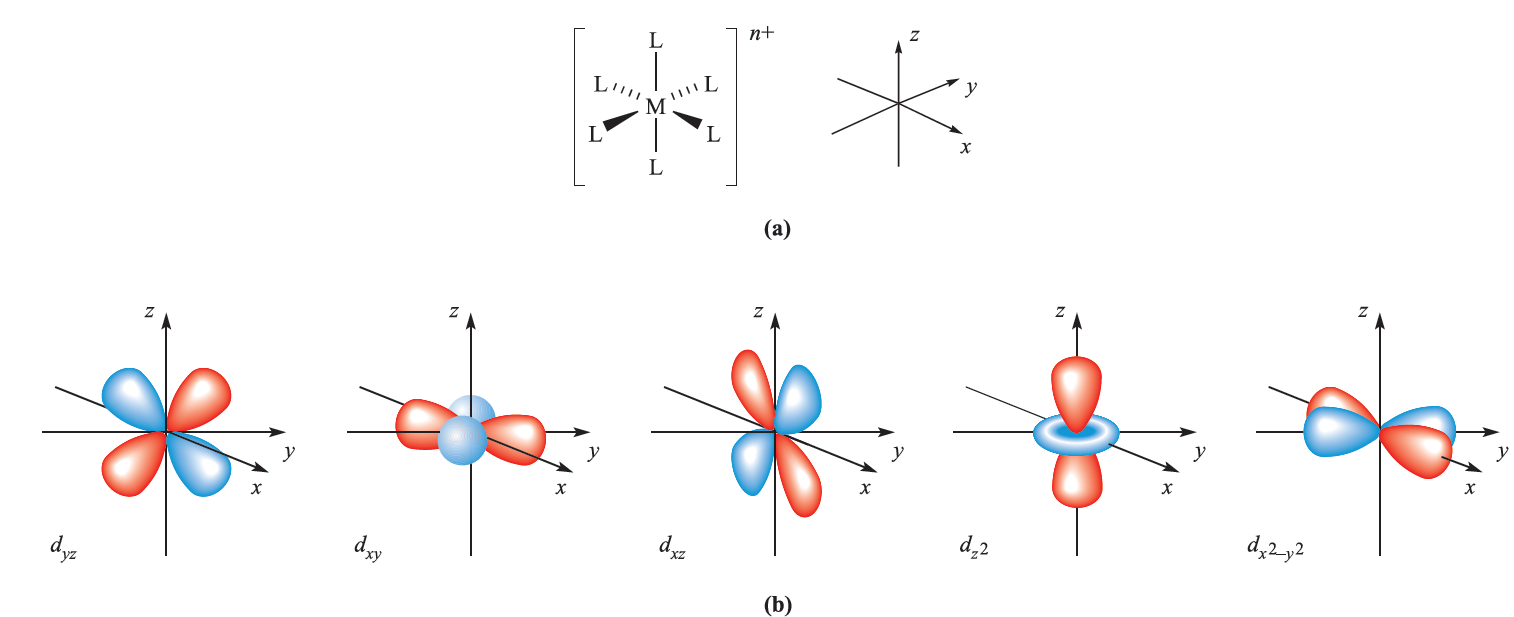
Fig. 1.1 (a) The six M_L vectors of an octahedral complex [ML6]n+ can be defined to lie along the x, y and z axes. (b) The five d orbitals; the dz2 and dx2- y2 atomic orbitals point directly along the axes, but the dxy, dyz and dxz atomic orbitals point between them.
Table 1.1 Hybridization schemes for the σ-bonding frameworks of different geometrical configurations of ligand donor atoms.



|
|
|
|
دراسة: حفنة من الجوز يوميا تحميك من سرطان القولون
|
|
|
|
|
|
|
تنشيط أول مفاعل ملح منصهر يستعمل الثوريوم في العالم.. سباق "الأرنب والسلحفاة"
|
|
|
|
|
|
|
لتعزيز التواصل مع الزائرات الأجنبيات : العتبة العلويّة المقدّسة تُطلق دورة لتعليم اللغة الإنجليزية لخادمات القسم النسويّ
|
|
|