


 الفيزياء الكلاسيكية
الفيزياء الكلاسيكية
 الكهربائية والمغناطيسية
الكهربائية والمغناطيسية
 علم البصريات
علم البصريات
 الفيزياء الحديثة
الفيزياء الحديثة
 النظرية النسبية
النظرية النسبية
 الفيزياء النووية
الفيزياء النووية
 فيزياء الحالة الصلبة
فيزياء الحالة الصلبة
 الليزر
الليزر
 علم الفلك
علم الفلك
 المجموعة الشمسية
المجموعة الشمسية
 الطاقة البديلة
الطاقة البديلة
 الفيزياء والعلوم الأخرى
الفيزياء والعلوم الأخرى
 مواضيع عامة في الفيزياء
مواضيع عامة في الفيزياء|
Read More
Date: 6-11-2016
Date: 10-10-2016
Date: 2-10-2016
|
Photon Engine
The ideal Carnot heat engine converts heat to work without the engine itself being a source of any work. The reversible closed Carnot cycle consists of two isothermal (constant temperature) processes and two adiabatic (no external exchange of thermal energy) processes. No heat engine operating between two temperatures can be more efficient than a Carnot cycle.
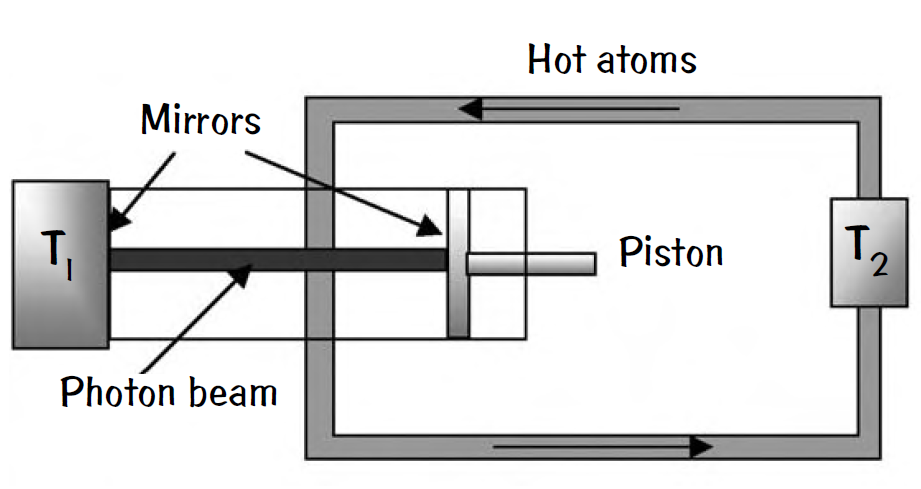
But Carnot could be wrong. The challenger is the new “quantum Carnot engine,” in which the radiation pressure from photons drives a piston in an optical cavity. The inward-facing surface of the piston is mirrored and the other cavity mirror is fixed in place while exchanging thermal energy with a heat sink at temperature T1. A second heat bath at a higher temperature, T2, provides the source of thermal energy for the photons.
This source of thermal energy is a stream of hot atoms, which flows into the optical cavity and exchanges thermal energy with the photons through emission and absorption processes. These atoms exit the cavity at a cooler temperature and are reheated to T2 in a second cavity, to be reinjected into the first cavity for the next cycle of the quantum Carnot engine.
Therefore, the quantum and classical Carnot engines operate in the same way as a closed cycle of two isothermal and two adiabatic processes. However, in its simplest form, when each bath atom is treated as a two-state system, the quantum Carnot engine cannot extract work from a single heat bath. Why not? Will the engine work if each bath atom is a three-state system?
Answer
We can analyze the operation of the quantum Carnot engine in the same manner in which we would analyze a classical Carnot engine. Let Qin be the energy absorbed from the bath atoms during the isothermal expansion and Qout be the energy given to the heat sink during the isothermal compression. Then the Carnot engine efficiency η = (Qin – Qout)/Qin.
If the bath atoms are assumed to be two-state systems that absorb and emit radiation at the same photon frequency, then we need the thermodynamic properties of a photon gas in order to determine the theoretical efficiency of this photon engine. Assuming thermal equilibrium for the photon gas, the average number of photons n2 with energy ε coming in from the heat bath at temperature T2 is given by n2 = 1/(exp[ε/kT2] – 1), while the average number of photons n1 leaving at temperature T1 is n1 = 1/(exp[ε/kT1] – 1). Since Qin ∝ n2 ε and Qout ∝ n1 ε, the efficiency of the quantum Carnot engine is η = 1 – T1/T2, exactly the same as for the classical Carnot engine. When there is only one heat bath, with T1 = T2, no work can be done.
A different quantum engine occurs when the bath atoms have three states instead of two, bringing in quantum behavior called quantum coherence, with a non-vanishing phase difference between the two lowest atomic states induced by a microwave field. One can eliminate the photon absorption process (analogous to laser operation without a population inversion). The temperature T2 becomes altered to a different effective temperature, Tφ. The efficiency ηφ = (Tφ – T1)/T1 can exceed the efficiency of the classical Carnot engine. This quantum engine can extract work from a single heat bath, even when T1 = T2! For the details of the three-state quantum engine’s operation, see the reference below.



|
|
|
|
دراسة: حفنة من الجوز يوميا تحميك من سرطان القولون
|
|
|
|
|
|
|
تنشيط أول مفاعل ملح منصهر يستعمل الثوريوم في العالم.. سباق "الأرنب والسلحفاة"
|
|
|
|
|
|
|
لتعزيز التواصل مع الزائرات الأجنبيات : العتبة العلويّة المقدّسة تُطلق دورة لتعليم اللغة الإنجليزية لخادمات القسم النسويّ
|
|
|