
The First Law of Thermodynamics
 المؤلف:
Professor John W. Norbury
المؤلف:
Professor John W. Norbury
 المصدر:
ELEMENTARY MECHANICS & THERMODYNAMICS
المصدر:
ELEMENTARY MECHANICS & THERMODYNAMICS
 الجزء والصفحة:
p 220
الجزء والصفحة:
p 220
 30-12-2016
30-12-2016
 2051
2051
The First Law of Thermodynamics
We have already studied this! The first law of thermodynamics is nothing more than a re-statement of the work energy theorem, which was
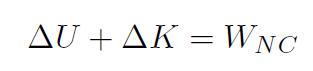
Recall that the total work W was always W = ΔK. Identify heat Q as Q ≡ WNC and internal energy (such as energy stored in a gas, which is just potential energy) is Eint ≡ U and we have
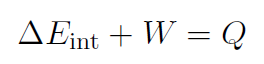
or
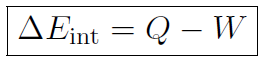
which is the first law of thermodynamics. The meaning of this law is that the internal energy of a system can be changed by adding heat or doing work. Often the first law is written for tiny changes as
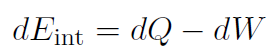
 الاكثر قراءة في الديناميكا الحرارية
الاكثر قراءة في الديناميكا الحرارية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


