علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Occurrence, extraction and uses Occurrence of group 14 elements
المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
المصدر:
INORGANIC CHEMISTRY
الجزء والصفحة:
2th ed p 338
23-3-2017
2178
Occurrence, extraction and uses Occurrence of group 14 elements
Figure 1.1 illustrates the relative abundances of the group 14 elements in the Earth’s crust. The two long-established crystalline allotropes of carbon, diamond and graphite, occur naturally, as does amorphous carbon (e.g. in coal).
Diamonds occur in igneous rocks (e.g. in the Kimberley volcanic pipes, South Africa). Carbon dioxide constitutes only 0.04% of the Earth’s atmosphere, and, although vital for photosynthesis, CO2 is not a major source of carbon.
During the 1990s, it was discovered that molecular allotropes of carbon, the fullerenes (see Section 13.4), occur naturally in a number of deposits in Australia, New Zealand and North America; however, laboratory synthesis remains the chief means of accessing these new allotropes. Elemental Si does not occur naturally, but it constitutes 25.7% of the Earth’s crust (Si is the second most abundant element after O) in the form of sand, quartz, rock crystal, flint, agate and silicate minerals . In contrast, Ge makes up only 1.8 ppm of the Earth’s crust, being present in trace amounts in a range of minerals (e.g. zinc ores) and in coal. The principal tin-bearing ore is cassiterite (SnO2). Important ores of lead are galena (PbS), anglesite (PbSO4) and cerussite (PbCO3).
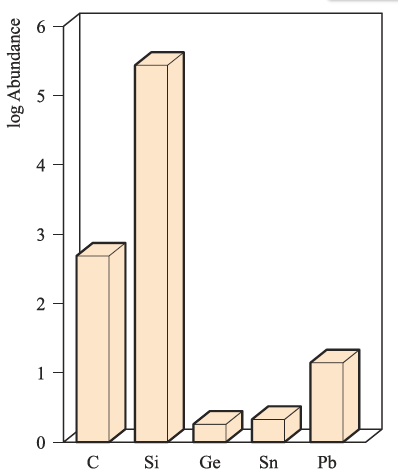
Fig. 1.1 Relative abundances of the group 14 elements in the Earth’s crust. The data are plotted on a logarithmic scale. The units of abundance are parts per million (ppm).
 الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
الاكثر قراءة في مواضيع عامة في الكيمياء اللاعضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)