
آخر المواضيع المضافة


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 25-2-2018
Date: 23-1-2017
Date: 2-5-2017
|
Stoichiometry of Reactions in Solution
Stoichiometric calculations involving solutions of specified molar concentration are usually quite simple since the number of moles of a reactant or product is simply volume × molar concentration.
Example 1
What volume of 1.40 M H2SO4 is needed to react exactly with 100 g of aluminum according to the Equation
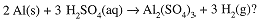
First calculate the number of moles of Al, then moles of H2SO4 needed and then the volume of H2SO4
solution required:
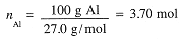

Example 2
40.0 mL of an H2SO4 solution was titrated with 0.215 M NaOH. 35.26 mL of base was required to
exactly neutralize H2SO4. What was the concentration of the acid?
The titration reaction,
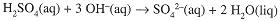
shows that 2 mol NaOH are needed to react with 1 mol H2SO4. Thus,




|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
دراسة تستعرض آلام السجناء السياسيين في حقبة البعث المجرم في العراق
|
|
|