


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 18-12-2020
Date: 30-9-2018
Date: 28-9-2018
|
Reaction Rates
Consider a general reaction,
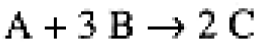 (1.1)
(1.1)
We describe the rate of the reaction in terms of the rate of disappearance of one of the reactants or the rate of appearance of the product,
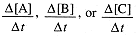
The stoichiometry of the reaction tells us that in time interval Δt, Δ[B] = 3 Δ[A], Δ[C] = - 2 Δ[A]; furthermore, Δ[A] and Δ[B] are negative, Δ[C] is positive. Thus we must include an appropriate sign and stoichiometric coefficient in expressing the rate of the reaction:
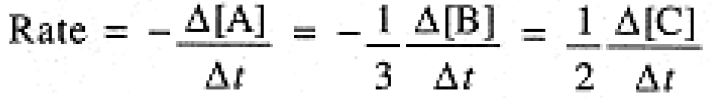 (1.2)
(1.2)
It is most common to express rates in terms of molar concentrations of species, even for gas-phase reactions. The usual units of a reaction rate are mol L-1s-1.
When a reaction is slow enough, it is appropriate to express the rate as the ratio of a concentration change, Δ[A], to a time interval, Δt, but when the reaction is faster, we consider the limit as Δt approaches zero, the first derivative of [A] with respect to time:
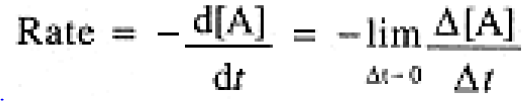 (1.3)
(1.3)



|
|
|
|
دراسة: حفنة من الجوز يوميا تحميك من سرطان القولون
|
|
|
|
|
|
|
تنشيط أول مفاعل ملح منصهر يستعمل الثوريوم في العالم.. سباق "الأرنب والسلحفاة"
|
|
|
|
|
|
|
سماحة السيد الصافي: السيد أحمد الإشكوري حجّةٌ على كلّ طالب علمٍ في عمره وإخلاصه وجهده
|
|
|