

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Vapour-phase synthesis of nanoparticles
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
الجزء والصفحة:
661-662
2025-10-13
52
Vapour-phase synthesis of nanoparticles
Key points: Vapour-phase synthetic methods are alternative techniques for nanoparticle synthesis because they have atomically mixed and highly mobile reagents, can be controlled by varying the conditions, and have been widely successful in practice. The same fundamentals concerning nucleation and growth that are relevant to solution synthesis apply to vapour-phase synthesis. The vapour needs to be supersaturated to the point at which a high density of homogeneous nucleation events produces solid particles in one short burst, and growth must be limited and controlled in a subsequent step, if it is to occur at all. Commercially, vapour-phase synthesis is carried out to produce nanoscale carbon black and fumed silica in large quantities. Metals, oxides, nitrides, carbides, and chalcogenides can also easily be formed by using vapour-phase techniques. There are, however, several differences between vapour-phase and solution-based techniques. In the latter, stabilizers can be added in a straightforward and controllable fashion, and particles remain dispersed and independent of one another. In vapour-phase techniques, however, surfactants or stabilizers are not easily added (although solid-state stabilizers are available). Without surface stabilizers, nanoparticles tend to agglomerate into larger particles. If temperatures during the process are high enough, the particles sinter and coalesce into one larger particle. Although the ‘soft agglomerates’ formed under the influence of weak intermolecular forces can usually be redispersed, the ‘hard agglomerates’ (partially sintered-formed at intermediate temperature) and coalesced particles (formed at high temperatures) cannot be. Therefore, techniques that lead to soft agglomerates are generally best suited to nanoparticle production. Because of these differences, the size dispersion of nanoparticles tends to be better for solution-based techniques than for vapourphase techniques. Vapour-phase techniques are attractive synthesis methods for particles when continuous operation is required or when solution methods do not produce good quality nanoparticles. They can also produce complex materials in a straightforward manner, such as doped binary and ternary compounds or composite particles. Vapour-phase techniques are classified by the physical state of the precursor used as a reagent and by the reaction method, such as ‘plasma synthesis’ or ‘flame pyrolysis’. In each case, the reagent is converted to a supersaturated or superheated vapour that is allowed to react or to cool to force nucleation. Solid reagents are vaporized and then recondensed in gas condensation methods (thermal evaporation to produce vapour), laser ablation, sputtering, and spark discharge methods. Liquid or vapour precursors are used in spray pyrolysis, flame synthesis, laser pyrolysis, plasma synthesis, and chemical vapour deposition. In chemical vapour deposition, the vapour is transported to a substrate where reaction and solid nucleation occurs (Fig. 25.5). In a variation of this procedure, gaseous precursors are delivered into a hot-walled reactor and allowed to react homogeneously in the vapour phase to nucleate solids, and particles are collected downstream. Particle sizes are controlled by the flow rates, precursor chemistry, concentrations, and residence times in the reactor. Examples of materials synthesized by this technique include metals, oxides, nitrides, and carbides such as SiC, SiO2, Si3N4, SiCxOyNz, TiO2, TiN, ZrO2, and ZrN. Both solution and vapour-phase methods can be used to make composite nanoparticles, such as core–shell nanocomposites. In both techniques the approach is to grow a second phase on an initial nucleus or nanoparticle. Reaction design to produce core– shell nanoparticles by solution is straightforward provided the solution characteristics of both materials are similar. In practice, however, it is difficult to find materials where the synthesis conditions overlap. Vapour-phase techniques offer another approach to the design of core–shell particles, by injecting a second vapour into a reactor at the growth stage. Another straightforward approach to vapour-phase nanoparticle production is plasma synthesis, which can be used to synthesize elemental solids, alloys, and oxides, as well as core–shell nanoparticles. In this method, gas or solid particles are fed into a plasma where they vaporize and ionize to highly energetic charged species. Inside a plasma, the temperatures can exceed 10 kK. On leaving the plasma, the temperature falls rapidly and crystallization occurs under conditions far from equilibrium. The nanoparticles are collected downstream from the plasma zone. Depending on the carrier gas (that is, the oxidation conditions), elemental solids, core–shell, or compound particles can be formed. Figure 25.6 shows two examples of nickel/zinc ferrite particles.
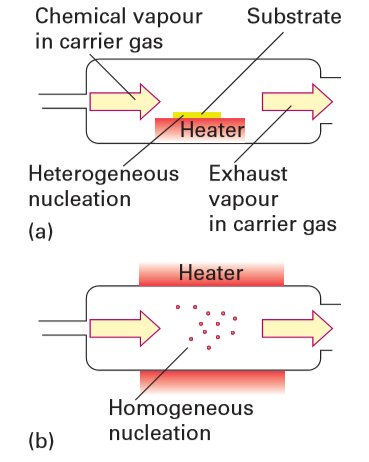
Figure 25.5 Chemical vapour methods to achieve (a) thin film growth and (b) nanoparticle production.
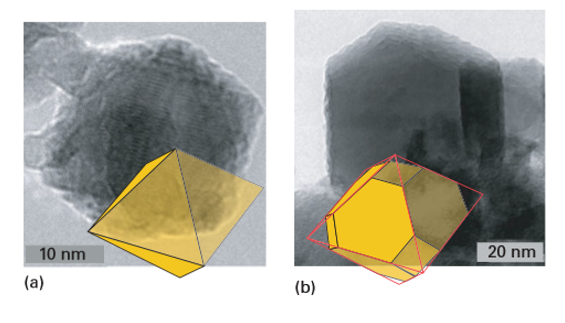
Figure 25.6 TEM images of nanoparticles of Ni0.5Zn0.5Fe2O4 synthesized by plasma torch synthesis. The particle shapes are superimposed: (a) an octahedron and (b) a truncated octahedron. (Based on images provided by M. McHenry and R. Swaminatham.)
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















