علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Physical vapour deposition
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
الجزء والصفحة:
664-665
2025-10-13
331
Physical vapour deposition
Key points: In the physical vapour deposition methods, a vapour of atoms, ions, or clusters physically adsorb to the surface and combine with other species to create a solid; molecular beam epitaxy is a technique where evaporated species from elemental charges are directed as a beam at a substrate where growth occurs. In physical vapour deposition (PVD) methods, vapours are delivered from their source to a solid substrate on which they crystallize. The arriving gaseous species are typically atoms, ions, or clusters of elements. There are several general forms of PVD that are widely used: molecular beam epitaxy (MBE), sputtering, and pulsed laser deposition (PLD). The gas-phase species can have either relatively low kinetic energies on arrival (as in MBE) or relatively high kinetic energies (as in sputtering and PLD). The most important feature that all PVD methods have in common is the ability to achieve complex film stoichiometries, either through the transfer of the different species between the source and the film or by using multisource systems to compensate for deviations from the ideal film composition. Because vapour deposition methods allow single atomic layers to be deposited in a controlled fashion on a support or substrate, nanoscale architectures can be built from the bottom up. In this section we describe some fundamental principles for these processes in systems that allow for real-time, in situ control over the nanostructure of materials. Molecular beam epitaxy is an ultrahigh vacuum technique for growing thin epitaxial films, films that have a definite crystallographic relationship with the underlying substrate. In MBE, molecular beams are formed by heating elemental sources until atoms evaporate and are transported ballistically to the substrate surface with relatively low kinetic energies (of the order of 1 eV). The overall pressure is kept very low to minimize collisions within the beam. To prevent species from bouncing off other surfaces of the system and back towards the substrate, much of the system is cooled with liquid nitrogen. In that way, stray elemental species adsorb onto surfaces and remain there. This cooling also helps to maintain the vacuum. Film stoichiometry and film growth rates are highly dependent on the beam fluxes, which can be controlled by adjusting the temperatures of the elemental sources. Homoepitaxy is the epitaxial growth of a thin film of a material on a substrate of the same material. Heteroepitaxy is the epitaxial growth of a thin film of a material on a substrate of a different material. Heteroepitaxy introduces a strain between the growing material and the substrate caused by their crystallographic mismatch. This strain can lead to a self-assembly process where nano-islands form in ordered arrays on the surface to minimize strain energies. By alternating the deposition of two materials by MBE, a multilayer structure of ordered QDs can be created (Fig. 25.9). By tailoring the structure of the substrate appropriately, nanowires and nanowells can also be created, as well as the superlattices and artificially layered crystals we describe later. Pulsed laser deposition is a versatile PVD technique that can be used to synthesize a wide variety of high-quality thin films (Fig. 25.10). In PLD, a pulsed laser is used to ablate a target, which releases a plume of atomized and ionized particles from its surface that condenses onto a nearby target. The PLD process usually produces films of composition identical to that of the substrate, which is a major simplification compared with techniques that require fine-tuning or expensive control equipment to achieve a specific stoichiometry. This simplicity makes PLD attractive for exploratory materials chemistry because the cation stoichiometry is controlled externally and independently of the deposition parameters. The technique also allows control over a number of deposition conditions that alter the thermodynamics and kinetics of growth, including substrate temperature, laser energy, and atmospheric conditions (partial pressures of O2, N2, or Ar); it can be carried out at pressures at or below 500 mTorr (about 100 Pa). Pulsed laser deposition can achieve average growth rates similar to those of MBE (for example, 100 pm s-1) but the instantaneous growth is much higher because growth occurs only in the short intervals (of about 1 µs) immediately after the 10-20 ns laser pulse ablates the target. Therefore, for a very short period there is a very high arrival rate and a very considerable supersaturation at the substrate surface, leading to very high nucleation rates. An additional difference from MBE is that in PLD the energies of the particles arriving at the substrate are usually 10–100 eV, which is large enough that there is the potential that arriving particles could damage the growing film. Nevertheless, PLD has been used to grow a variety of high-quality superlattices, such as the SrMnO3 and PrMnO3 superlattice film shown in Fig. 25.11 and described later.
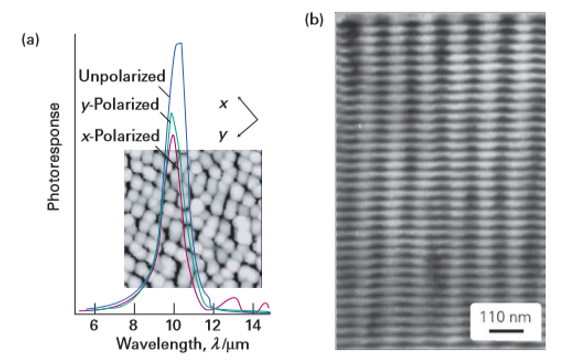
Figure 25.9 (a) Photoresponse (in arbitrary units) of (In0.2Ga0.8) As quantum dots formed using MBE. The IR absorption band arises from intraband absorption. The inset is an AFM image of the quantum dots. The quantum dots vary in lateral dimensions between 15 and 20 nm and in vertical height in the range 3–7 nm. (b) A crosssectional TEM micrograph of an ordered superlattice of (In0.2Ga0.8) As quantum dots in a GaAs matrix. (Courtesy of E. Towe.)
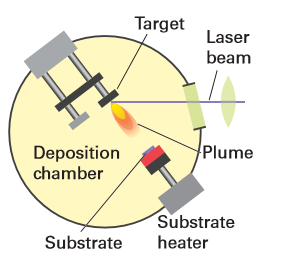
Figure 25.10 A pulsed laser deposition chamber used to produce nanostructured super lattices and artificially layered thin films.
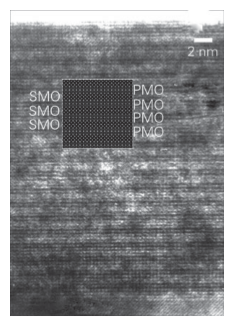
Figure 25.11 Cross-sectional TEM image of a 2 x 2 superlattice of SrMnO3 and PrMnO3 prepared using laser-MBE. The inset shows a calculated image confirming the (SrMnO3)2(PrMnO3)2 structure. (Reprinted with permission from B. Mercey, et al., In situ monitoring of the growth and characterization of (PrMnO3)n (SrMnO3)n superlattices. J. Appl. Phys., 2003, 94, 2716, American Institute of Physics.)
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)