علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Solid-state superlattices
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
الجزء والصفحة:
ص673-675
2025-10-15
59
Solid-state superlattices
Key points: Artificially layered materials have a periodic repeat along the growth direction of a thin film; the periodic repeat is controlled by the number and type of sublayers deposited in sequence. The periodic repeat along the growth direction of a thin film in a superlattice is controlled by the number and type of sublayers deposited in sequence, whereas the lateral periodicity is determined by the coherency—the matching of lattice characteristics—between the
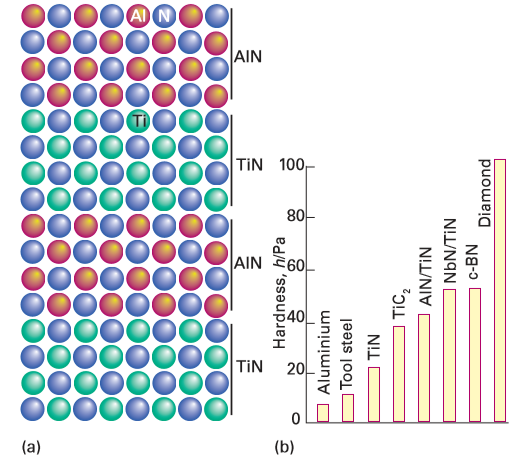
Figure 25.22 (a) The structure of an ultrahard AlN/TiN superlattice and (b) the hardness of a nitride superlattice and commonly used hard materials. (Adapted from S.A. Barnett and A. Madan, Physics World, 1998, 11, 45.)
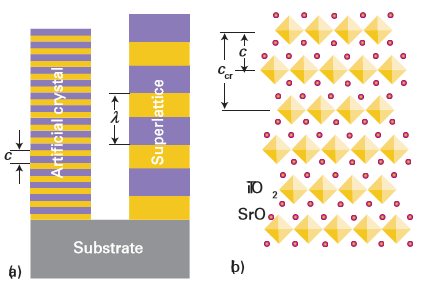
Figure 25.23 (a) The structure of an AB artificial crystal and an AB superlattice; c and λ represent the repeat period during growth of the artificial structure and the superlattice period, respectively. (b) The structure of Sr2TiO4 as an artificially layered oxide. The polyhedra represent Ti-centred, corner-sharing TiO6 octahedra and the spheres represent Sr2+ cations. The lateral coherency between the SrO and TiO2 sublayers is excellent in this layered structure and the crystallographic repeat parameter, ccr, is twice the growth repeat period, c.
sublayers (Figures 25.22 and 25.23). It should be kept in mind that the superlattice is built bottom-up with periods in the nanometre range and total thicknesses in the micrometre range. The goal of materials chemists and engineers is to use these synthesis skills to control the properties and functionality of materials of this kind. Superlattice nitrides are ranked among the hardest known materials. The superlattice period and the chemical composition play important roles in determining the mechanical properties of such compounds, as the interfaces between the two nitride layers are responsible for the enhanced hardness. Figure 25.24 shows, for instance, that the maximum hardness of a typical superlattice occurs for periodicities in the range 5–10 nm. These superlattices have been successfully deposited using sputtering, pulsed laser deposition, and molecular beam epitaxy. Such superlattices are used on cutting tools and are made economically by using sputtering to form them. Perovskite oxides (Section 24.7) are used in numerous applications on account of their ferroelectric, acoustic, microwave, electronic, magnetic, and optical properties. The most widely used perovskite is BaTiO3, which is a very important dielectric material. It has been discovered that layering ferroelectric BaTiO3 with the isostructural perovskite SrTiO3 can lead to enhanced dielectric properties arising from lattice strain. The techniques of PLD, MBE, and CVD have all been used to create SrTiO3/BaTiO3 superlattice thin films (Fig. 25.25). Although the misfit between the two crystal structures is small enough to allow for epitaxial growth and the formation of coherent interfaces between each bilayer, the stress introduced at the interface is sufficient to improve the dielectric response, specifically the remanent polarization (the polarization in the absence of an applied field) of the superlattices, compared to undoped BaTiO3.
It is important to control layer thickness precisely to ensure the formation of coherently strained interfaces that lead to improved dielectric response. The technique of PLD has been used to deposit alternating blocks of SrTiO3 and BaTiO3 having unequal layer thicknesses of SrTiO3/BaTiO3 15/3, 10/3, 3/10, and 3/15, and the net dipole moment density of superlattices has been reported to be in the order of 46 µC cm-2, whereas that of bulk films of the parent phases is about 14 µC cm-2. Perovskite-based structures can also exhibit interesting magnetic effects. In particular, manganese-based perovskite films such as (La,Sr)MnO3 possess useful ferromagnetic and magnetoresistive properties. In general, these characteristics arise from electron exchange interactions occurring between Mn and O that are mediated by the various cations in the structure, and can be altered significantly when these cations are spatially ordered. The PLD procedure has been used to deposit superlattices of LaMnO3/SrMnO3; the Mn3cations are found in the LaMnO3 layers and the Mn4+ are found in the SrMnO3 layers. Thus, the thin-film superlattice technique allows for precise ordering of A-site cations (La, Sr), which in turn causes ordering of the Mn in its different oxidation states. In superlattices of (LaMnO3) m(SrMnO3) m, samples with m ≤ 4 possess magnetic properties just like solid solutions of La0.5Sr0.5MnO3, whereas superlattices with larger periods have significantly higher resistivities and lower Curie temperatures. Similar effects are observed in superlattices with unequal layer thicknesses, such as (LaMnO3) m(SrMnO3)n and (PrMnO3)m(SrMnO3)m systems (Fig. 25.11).
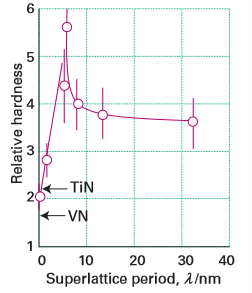
Figure 25.24 The dependence of hardness on the superlattice period for (TiN)m(VN)m superlattices. (Adapted from U. Helmersson, et al., J. Appl. Phys., 1987, 62, 481.)
The magnetic properties of thin-film superlattices in the family La (Sr)MnO3/LaMO3 (M= Fe, Cr, Co, and Ni) exhibit a wide range of properties depending on both superlattice repeat period and the identity of M. For instance, when M = Co or Ni the Curie temperature is increased but when M Fe or Cr it is decreased. Interesting electronic effects are observed in superlattice thin films consisting of spinel Fe3O4 and rock-salt NiO layers. Superlattices of these two structures are well matched because both have the same O sublattice and differ only in the locations of the cations. Superlattices of composition Fe3O4/NiO have been grown by MBE for a variety of layer thicknesses. The electronic conduction mechanism in many d-metal oxides such as these is electron hopping, a process that has a short mean free path. If this distance is approximately the same as the crystal lattice spacings it can lead to novel electrical properties for artificially layered materials with short lattice spacings. Thus, Fe3O4/NiO superlattices with bilayer repeat periods of 6.8 nm have electrical conductivities that differ by factors in the range 106–108 in different directions, which is among the highest known electrical anisotropy for any material. This result effectively demonstrates both the high degree of chemical modulation available by superlattice deposition and the extent of electrical conduction localization that can be achieved as a consequence.
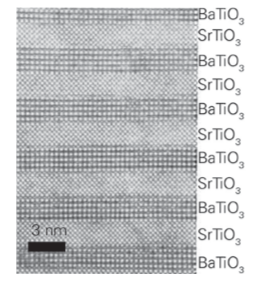
Figure 25.25 TEM image of a SrTiO3 / BaTiO3 superlattice. (Reprinted from D. G. Schlom, et al., Oxide nano-engineering using MBE. Mater. Sci. Eng. B, 2001, 87, 282, with permission from Elsevier.)
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)