علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Bionanocomposites
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
الجزء والصفحة:
ص682-687
2025-10-15
61
Bionanocomposites
Key points: Bionanomaterials synthesis offers new routes to realize smart materials with advanced mechanical strengths and improved performance over natural materials; protein engineering offers an opportunity to insert species that attach selectively to inorganic materials.

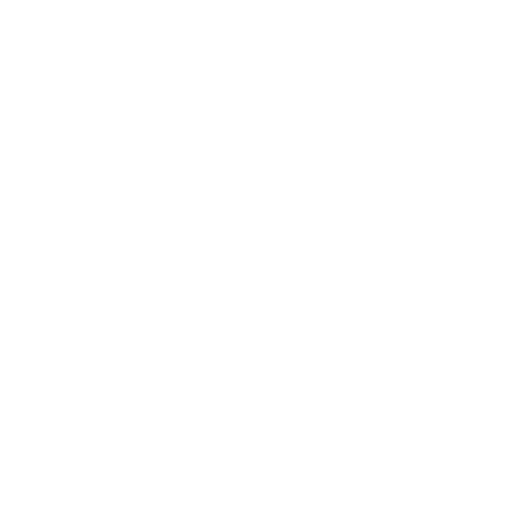
Millions of bone fractures resulting in hospitalization occur worldwide every year. Modern treatments for severe bone injuries replace a nonuniform defect with a permanent biomaterial that may corrode, wear, and ultimately cause severe infection. In addition, these biomaterials typically exhibit mechanical strength far greater than that of bone, resulting in stress shielding and eventual bone resorption around the implant. There is a social need for a bone tissue engineering scaffold that has mechanical properties similar to bone and that will facilitate bone growth into the defect, degrade slowly and naturally, and eventually be replaced by natural bone tissue without threat of infection. The primary obstacle towards this goal is matching the unique mechanical properties of natural bone tissue with a degradable, synthetic nanomaterial. The versatile and important structural properties of bone are derived from the nanoscale interactions between its inorganic and organic components. There are two types of bone, trabecular bone, the weaker and porous central part, and cortical bone, the stronger and denser outer shell of bone. Hydroxyapatite nanocrystals provide compressive strength as they precipitate onto bundles of collagen fibres that impart tensile strength. Degradable biomaterials composed of inorganic and organic components can have mechanical properties closely matching natural bone tissue, and they may be suitable for load-bearing bone tissue engineering applications. Here we describe one illustrative example of these approaches that combines polymeric components with inorganic components.
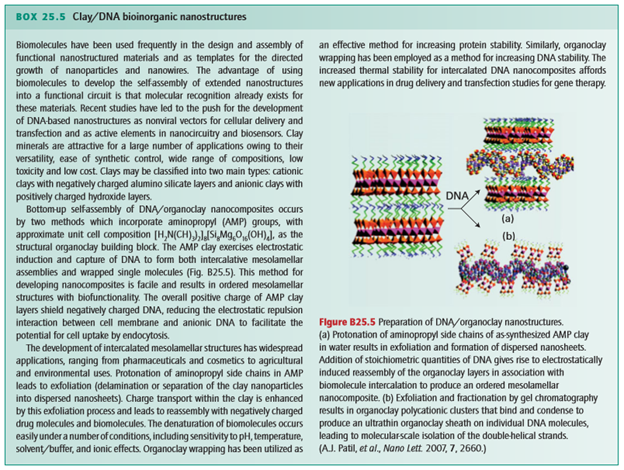
Poly(propene fumarate) (PPF) cross-linked with poly(propene fumarate)-diacrylate (PPF-DA) is an injectable, biodegradable material. Although PPF shows great potential as a material for bone tissue engineering where mechanical properties such as tensile strength are not crucial, such as the trabecular bone, its mechanical properties are inferior to those required for the more demanding human bone applications, such as cortical bone. A promising strategy to improve the mechanical properties of a polymer is to mimic the architecture of bone or to incorporate inorganic particles into the polymer matrix. For example, significant increases have been observed in the compressive mechanical properties of PPF by the incorporation of calcium phosphate. The extent to which inorganic particles modify the polymer properties is associated with their size, shape, and uniformity of dispersion, as well as with the degree of interaction between the inorganic and organic components (similar to the red abalone shell, Box 25.4). Chemically modified inorganic materials that allow for optimal dispersion and interaction with the polymer matrix therefore seem to be ideal candidates for use in bionanocomposites. A hybrid alumoxane nanoparticle, or a partially hydrolysed aluminium oxyhydroxide with surrounding organic material (Fig. 25.39), with a long carbon chain and a reactive double bond dispersed in PPF/PPF-DA, shows over a threefold increase in strength over polymer resin alone. Several approaches have been successful for attaching nucleic acids to nanoparticles, which is then followed by DNA hybridization like that mentioned in Section 25.10. Various functional inorganic nanomaterials, such as semiconductors, fullerenes, metals, and oxides, have been considered for use in bottom-up approaches to the design of systems that bridge inorganic and biological materials. One approach is to functionalize the surface of nanoparticles for selective molecular attachment; such materials are used in the development of biosensors, nanoprobes, drug transporters, and other smart devices (Box 25.5).
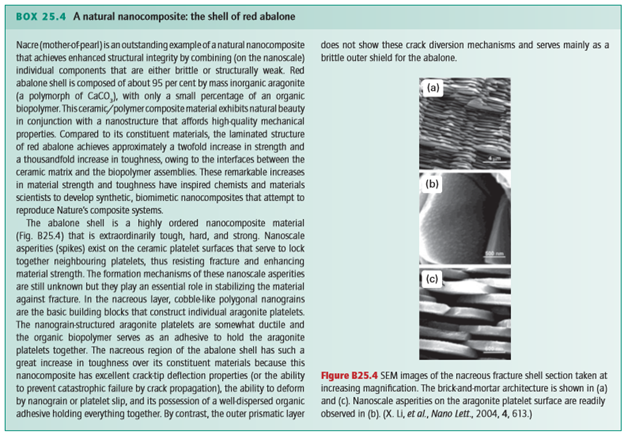
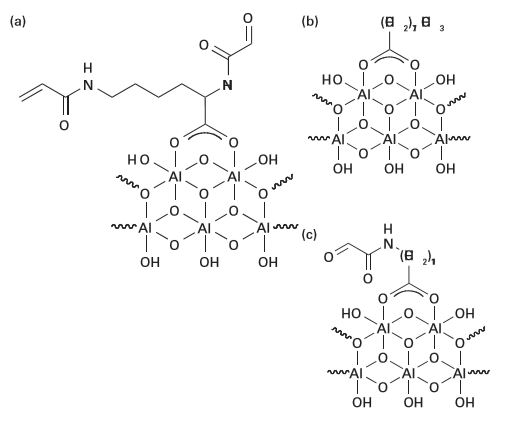
Figure 25.39 Chemical structures of modified alumoxanes: (a) diacryloyl lysine– alumoxane (activated), (b) stearic acid– alumoxane (surfactant), and (c) acryloyl undecanoic amino acid–alumoxane (hybrid). (R.A. Horch, et al., Biomacromolecules, 2004, 5, 1990.) (Reproduced with permission from the American Chemical Society.)
Proteins have been bound to nanoparticles by using a single site on the protein to yield hybrid nanomaterials. An important example of this type of material consists of proteins attached to fullerenes, which take advantage of the latter’s rich chemical and electronic properties, small dimensions, and potential applications. Alterations to the amino acid sequence of a protein are carried out by site-directed mutagenesis (SDM). By using SDM, a single cysteine residue, which selectively attaches to the fullerene, has been introduced in the protein sequence away from the active site to avoid deactivation on binding to the nanoparticles. Figure 25.40 illustrates one approach to create a covalent linkage between nanosized fullerenes and an active protein by using a functional tether (a bridging molecule). Many of these functionalized fullerenes have found biological applications as inhibitors of HIV protease, as light-sensitive biochemical probes, and in photodynamic tumour therapies. Engineering covalent linkages between nanomaterials and proteins has outstanding potential for future applications in drug delivery and enhanced drug efficacy. There is a new world of inorganic chemistry, as we have sought to show, waiting in the wings of our subject: this intermediate domain between the atomic and the bulk is waiting for discovery and exploitation. With exploitation comes the responsibility of careful consideration of the effects that nanomaterials will have on our environment both as novel materials for water filtration and remediation of biohazards and as sources of toxic pollutants (Box 25.6). It is essential to have a reliable and extensive grounding in the conventions of inorganic chemistry, to note what Nature has already achieved, and—above all—to build on it with imagination.
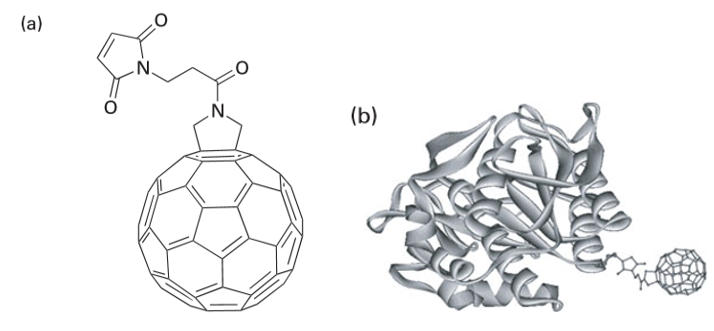
Figure 25.40 (a) Structure of N-(3maleimidopropionyl)-3,4-fulleropyrrolidine. (b) The protein conjugated to N-(3maleimidopropionyl)-3,4-fulleropyrrolidine (shown to scale). (P. Nednoor, et al., Biocon. Chem., 2004, 15, 12.)
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)