

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
The reactions of cobalt-containing enzymes
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
الجزء والصفحة:
ص759-763
2025-10-26
332
The reactions of cobalt-containing enzymes
Key points: Nature uses cobalt in the form of complexes with a macrocyclic ligand, known as corrin. Complexes in which the fifth ligand is a benzimidazole that is covalently linked to the corrin ring are known as cobalamins. Cobalamin enzymes catalyse methyl transfer and dehalogenation.

Figure 27.40 The S-cycle for evolution of O2 by successive one-electron oxidations of the [4MnCa 4O] cluster of photosystem II. The formal oxidation numbers of the Mn components are indicated, but H+ transfers are omitted. In chloroplasts that have become adapted to dark conditions, the cycle ‘rests’ in the S1 state.
In Coenzyme B12 the sixth ligand is deoxyadenosine, which is coordinated through a Co-C bond; en zymes containing coenzyme B12 catalyse radical-based rearrangements.
Cobalt macrocycle complexes are cofactors in enzymes that catalyse methyl transfer reactions and they are also important for dehalogenation and radical-based rearrangements (for example, isomerizations). The macrocycle is a corrin ring (9), which is similar to porphyrin (8), except there is less conjugation and it has a smaller ring (15-membered instead of 16-membered). The five-coordinate complex known as cobalamin includes a fifth nitrogen donor in one of the axial positions: usually this ligand is a dimethylbenzimidazole that is covalently linked to the corrin ring through a nucleotide, but a histidine residue is also commonly encountered. The more elaborate structure known as coenzyme B12 (44) is an important enzyme cofactor for radical rearrangements: the sixth ligand, R, is 5-deoxya denosine, which is bonded to the Co atom through the -CH2- group, making coenzyme B12 a rare example of a naturally occurring organometallic compound.8 The sixth ligand is exchangeable and the complex is ingested in the form of species such as aquacobalamin, hydroxocobalamin, or cyanocobalamin, known generally as vitamin B12. Cobalamin is essential for higher organisms (the human requirement is only a few milligrams per day) but it is synthesized only by microorganisms. Like Fe porphyrins, the Co corrins are enzyme cofactors and exert their activities when bound within a protein.
The Co atom can exist in three oxidation states under physiological conditions, Co (III), Co (II), and Co(I), all of which are low spin. The electronic structure of Co is crucial to its biological activity. As expected, the Co (III) form (d6) is an 18-electron, six-coordinate species (45). The Co (II) form (46) is 17-electron, five-coordinate and has its unpaired electron in the dz2 orbital. These species are termed ‘base-on’ forms because the fifth nitrogen ligand is coordinated. The Co(I) form (47) is a classic 16-electron, four-coordinate square planar species, due to dissociation of both axial ligands. The square-planar structure is a ‘base-off’ form.

8Coenzyme B12 was one of the earliest molecules to be structurally characterised by X-ray diffraction methods (Dorothy Crowfoot Hodgkin, Nobel Prize for Chemistry, 1964). In 1973, Robert Woodward and Albert Eschenmoser published the total synthesis of B12, the most complex natural product to be synthesized at that time, involving almost 100 steps.
Methyl transfer reactions of cobalamins exploit the high nucleophilicity of square-planar Co(I). A particularly important example is methionine synthase, which is responsible for the biosynthesis of methionine. Methionine is produced by transferring a CH3 group, de rived from the methyl carrier methyl hydrofolate, to homocysteine. Not only is methionine an essential amino acid, but also accumulation of homocysteine (which occurs if activity is impaired) is associated with serious medical problems. The mechanism involves a ‘base on/base-off’ cycle in which Co(I) abstracts an electrophilic CH3 group (effectively CH3) from a quaternary N atom on N5-tetrahydrofolate to produce methylcobalamin, which then transfers CH3 to homocysteine (Fig. 27.41). Methylcobalamin is the methyl-trans ferring cofactor for a wide variety of biosynthetic pathways, including the production of antibiotics. In anaerobic microbes, methylcobalamin is involved in the synthesis of acetyl coenzyme A, an essential metabolite, and in production of methane by methanogens. Radical-based rearrangements catalysed by coenzyme B12 (but see Box 27.1) include isomerizations (mutases) and dehydration or deamination (lyases). The generic reaction is

Dehydration and deamination occur after two -OH or -OH and -NH2 become placed on the same carbon atom and hence are triggered by isomerization:

Radical-based rearrangements occur by a mechanism involving initiation of radical formation that begins with enzyme-induced weakening of the Co C (adenosine) bond. In the free state, the Co C bond dissociation energy is about 130 kJ mol 1, but when bound in the
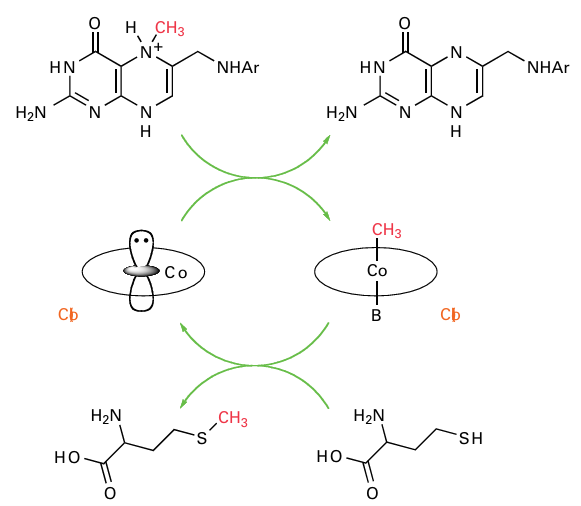
Figure 27.41 The mechanism of methionine synthase. Co(I) is a strong nucleophile and attacks the electrophilic quaternary-CH3 group on the methyl carrier tetrahydrofolate. The resulting Co (III) methyl complex transfers CH3+ to homocysteine.

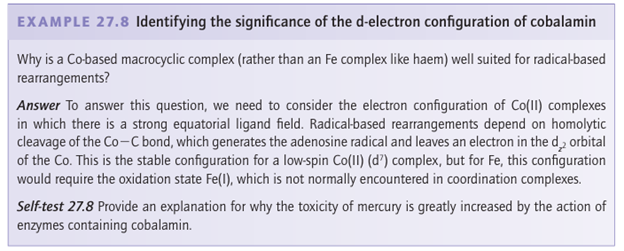
enzyme the bond is substantially weakened, resulting in homolytic cleavage of the Co-CH2R bond. This step results in five-coordinate low-spin Co (II) and a CH2 R radical, which gives rise to controlled radical chemistry in the enzyme active site pocket (Fig. 27.42). Important examples are methylmalonyl CoA mutase and diol dehydratases. In 1970, an alternative system for catalyzing radical-based rearrangements was discovered that does not depend on Co. As described in Box 27.1, the enzymes involved use instead a [4Fe-4S] cluster to generate the active deoxyadenosyl radical by reductive cleavage of its methionyl derivative.

Figure 27.42 The principle of radical-based rearrangements by coenzyme B12. Homolytic cleavage of the Co-C bond results in low-spin Co (II) (dz2 1) and a carbon radical that abstracts an H atom from the substrate RH. The substrate radical is retained in the active site and undergoes rearrangement before the hydrogen atom transfers back.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















