
Iron proteins as sensors
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص769-771
الجزء والصفحة:
ص769-771
 2025-10-26
2025-10-26
 46
46
Iron proteins as sensors
Key point: Organisms use sophisticated regulatory systems based on Fe-containing proteins to adapt quickly to changes in cellular concentrations of Fe and O2. We have already seen how FeS clusters are used in electron transfer and catalysis (Sections 27.8 and 27.9). The coordination of an FeS cluster ties together different parts of a protein and thus controls its tertiary structure. The sensitivity of the cluster to oxygen, electrochemical potential, or Fe and S concentrations makes it able to be an import ant sensory device. In the presence of O2 or other potent oxidizing agents, [4Fe 4S] clusters have a tendency (controlled by the protein) to degrade, producing [3Fe 4S] and [2Fe 2S] species. The cluster may be removed completely under some conditions (Fig. 27.48). The principle behind an organism’s exploitation of FeS clusters as sensors is that the presence or absence of a particular cluster (the structure of which is very sensitive to Fe or oxygen) alters the conformation of the protein and determines its ability to bind to nucleic acids. In higher organisms, the protein responsible for regulating Fe uptake (transferrin) and storage (ferritin) is an FeS protein known as the iron regulatory protein (IRP), which is closely related to aconitase (Section 27.9) but is found in the cytoplasm rather than in mitochondria. It acts by binding to specific regions of messenger RNA (mRNA) that carry the genetic command (transcribed from DNA) to synthesize transferrin receptor or ferritin. A specific interaction region on the RNA is known as the iron-responsive element (IRE). The principle is outlined in Fig. 27.49. When Fe levels are high, a [4Fe-4S] cluster is present, and the protein does not bind to the IRE that controls translation of ferritin. In this case binding would be a ‘stop’ command, and the cell will respond by synthesizing ferritin. Simultaneously, binding of the [4Fe 4S]-loaded protein to the transferrin receptor IRE destabilizes the RNA, so transferrin receptor is not made. When Fe levels are high, the opposite actions occur: a [4Fe-4S] cluster is formed, ferritin synthesis is activated, and transferrin receptor synthesis is switched off (repressed). The common gut bacterium E. coli derives energy either by aerobic respiration (using a terminal oxidase related to cytochrome c oxidase, Section 27.10) or by anaerobic respiration with an oxidant such as fumaric acid, or nitrate, using the Mo enzyme nitrate
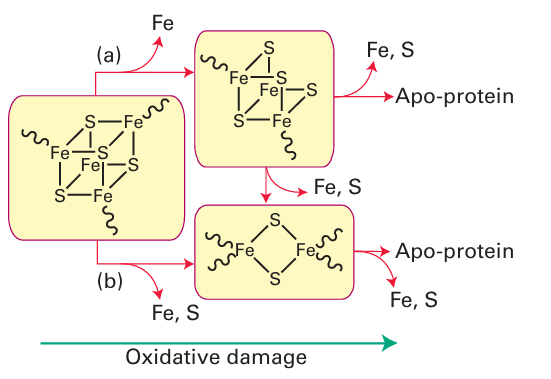
Figure 27.48 The degradation of Fe-S clusters forms the basis for a sensory system The [4Fe 4S] cluster cannot support a state in which all Fe are Fe (III); thus severe oxidizing conditions, including exposure to O2 , causes their breakdown to [3Fe-4S] or [2Fe-2S] and eventually complete destruction. Degradation to [3Fe-4S] (a) requires only removal of an Fe subsite whereas degradation to [2Fe-2S] (b) may require rearrangement of the ligands (cysteine) and produce a significant protein conformational change. These processes link cluster status to availability of Fe as well as O2 and other oxidants and provide the basis for sensors and feedback control.

Figure 27.49 Interactions of iron-regulatory protein with iron-responsive elements on the RNAs responsible for synthesizing ferritin or transferrin receptor depend on whether an Fe S cluster is present and form the basis for regulation of cellular Fe levels. reductase (Sections 27.12 and 27.13). The problem the organism faces is how to sense whether O2 is present at a sufficiently low level to warrant inactivating the genes functioning in aerobic respiration and activate instead the genes producing enzymes necessary for the less efficient anaerobic respiration. This detection is achieved by an FeS protein called fumarate nitrate regulator (FNR). The principle is outlined in Fig. 27.50. In the absence of O2, FNR is a dimeric protein with one [4Fe–4S] cluster per subunit. In this form it binds to specific regions of DNA repressing transcription of the aerobic enzymes and activating transcription of enzymes such as nitrate reductase. When O2 is present, the [4Fe-4S] cluster is degraded to a [2Fe 2S] cluster and the dimer breaks up so that it cannot bind to DNA. The genes encoding aerobic respiratory enzymes are thus able to be transcribed, whereas those for anaerobic respiration are repressed. In higher animals, the system that regulates the ability of cells to cope with O2 shortage involves an Fe oxygenase. Prolyl hydroxylases catalyse the hydroxylation of specific pro line residues in proteins, thus altering their properties. In higher animals, one such target protein is a transcription factor called hypoxia inducible factor (HIF), which mediates the expression of genes responsible for adapting cells to low-O2 conditions (hypoxia). We should bear in mind here that the internal environment of cells and cell compartments is usually quite reducing, equivalent to an electrode potential below –0.2 V, and even though we regard O2 as essential for higher organisms, its actual levels may be fairly low. When O2 levels are above a safe threshold, prolyl hydroxylases catalyse oxygenation of two conserved proline residues of HIF, causing the transcription factor to be recognized by a protein that induces its degradation by proteases. Hence genes such as those ultimately responsible for producing more red blood cells (which will help an individual to cope better when O2 supply is a problem) are not activated. The principle is outlined in Fig. 27.51. Although essential for higher organisms, O2 requires stringent control of its four-electron reduction, and increasing amounts of research are being carried out to prevent and cure malfunctions of normal O2 consumption. The term ‘oxidative stress’ is used to describe conditions in which the normal function of an organism is threatened by a build up of partially reduced O2 intermediates, such as superoxides, peroxides, and hydroxyl radi cals known collectively as reactive oxygen species (ROS). Prolonged exposure to ROS is associated with premature aging and certain cancers. To avoid or minimize oxidative stress cells must first sense ROS and then produce agents to destroy them. Both sensory and attack agents are proteins with active groups such as metal ions (particularly Fe, Cu) and exposed, redox-active cysteine thiols. An underlying principle of haem sensors is that the small molecules being sensed are π acceptors that can bind strongly to the Fe and displace an indigenous ligand. This bind ing results in a change in conformation that alters catalytic activity or the ability of the protein to bind to DNA. Of the indigenous ligands, two classic examples are NO and CO; although we have long thought of these molecules as toxic to higher life forms, they are becoming well established as hormones. Indeed, there is evidence that the sensing of trace levels of CO is important for controlling circadian rhythms in mammals. The enzyme guanyl cyclase senses NO, a molecule that is now well established as a hormone that delivers messages between cells. Guanyl cyclase catalyses the conversion of guanidine monophosphate (GMP) to cyclic GMP (cGMP), which is important for activating many cellular processes. The catalytic activity of guanyl cyclase increases greatly (by a factor of 200) when NO binds to the haem, but (as is obviously important) by a factor of only 4 when CO is bound. An excellent, atomically defined example of CO sensing is provided by a haem- containing a transcription factor known as CooA. This protein is found in some bacteria that are able to grow on CO as their sole energy source under anaerobic conditions. Whether growth on CO takes place depends on the ambient CO level, as an organism will not waste its resources synthesizing the necessary enzymes when the essential substrate is not present. CooA is a dimer, each subunit of which contains a single b-type cytochrome (the sensor) and a ‘helix-turn-helix’ protein fold that binds to DNA (Fig. 27.52). In the absence of CO, each Fe (II) is six-coordinate and both its axial ligands are amino acids of the pro tein, a histidine imidazole and, unusually, the main-chain NH2 group of a proline that is also the N-terminal residue of the other subunit. In this form, CooA cannot bind to the specific DNA sequence to transcribe the genes for synthesizing the CO-oxidizing enzymes necessary for existing on CO. When CO is present it binds to the Fe, displacing the distal proline residue and causing CooA to adopt a conformation that will bind to the DNA. The likelihood of NO binding in place of CO to cause a false transcriptional response is prevented because NO not only displaces proline but also results in dissociation of the proximal histidine; the NO complex is thus not recognized.
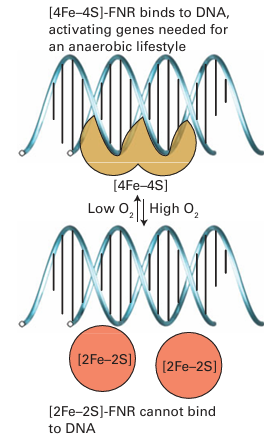
Figure 27.50 The principle of operation of the fumarate nitrate regulatory system that controls aerobic versus anaerobic respiration in bacteria.
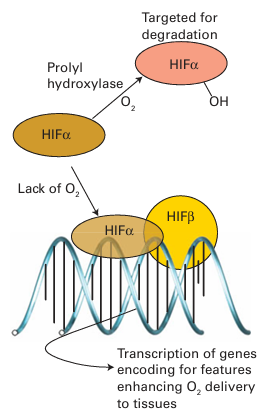
Figure 27.51 The principle of O2 sensing by prolyl oxygenases.

Figure 27.52 The structure of CooA, a bacterial CO sensor and transcription factor. The molecule, a dimer of two identical subunits (represented as red and blue), has two haem-binding domains and two ‘helix turn–helix’ domains that recognize a section of DNA. The protein ligands to the Fe atoms are a histidine from one subunit and an N-terminal proline from the other. The binding of CO and displacement of proline disrupts the assembly and allows CooA to bind to DNA.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


