
Solution-Diluent Volume Ratios
 المؤلف:
D. A. Skoog, F. J.Holler, D M. West, and S. R. Crouch
المؤلف:
D. A. Skoog, F. J.Holler, D M. West, and S. R. Crouch
 المصدر:
Fundamentals of Analytical Chemistry
المصدر:
Fundamentals of Analytical Chemistry
 الجزء والصفحة:
9th ed - p72
الجزء والصفحة:
9th ed - p72
 24-8-2016
24-8-2016
 1358
1358
Solution-Diluent Volume Ratios
The composition of a dilute solution is sometimes specified in terms of the volume of a more concentrated solution and the volume of solvent used in diluting it. The volume of the former is separated from that of the latter by a colon. Thus, a 1:4 HCl solution contains four volumes of water for each volume of concentrated hydrochloric acid. This method of notation is frequently ambiguous in that the concentration of the original solution is not always obvious to the reader. Moreover, under some circumstances 1:4 means dilute one volume with three volumes. Because of such uncertainties, you should avoid using solution-diluent ratios.
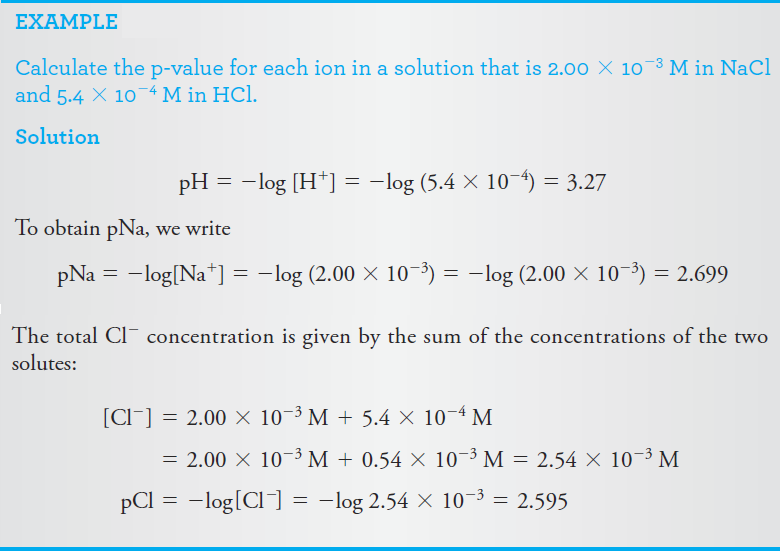
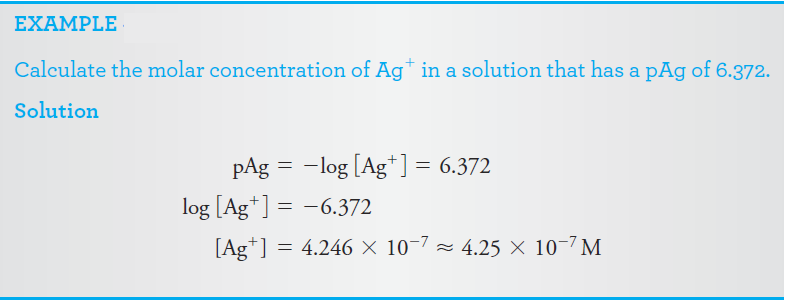
p-Functions
Scientists frequently express the concentration of a species in terms of its p-function, or p-value. The p-value is the negative logarithm (to the base 10) of the molar concentration of that species. Thus, for the species X,
pX = -log [X]
As shown by the following examples, p-values offer the advantage of allowing concentrations that vary over ten or more orders of magnitude to be expressed in terms of small positive numbers.
 الاكثر قراءة في التحليل النوعي والكمي
الاكثر قراءة في التحليل النوعي والكمي
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


