
Coulomb’s Law
 المؤلف:
المؤسسة العامة للتعليم الفني والتدريب المهني
المؤلف:
المؤسسة العامة للتعليم الفني والتدريب المهني
 المصدر:
مقدمة عن علم الكهربية الساكنة
المصدر:
مقدمة عن علم الكهربية الساكنة
 الجزء والصفحة:
...
الجزء والصفحة:
...
 17-2-2017
17-2-2017
 3501
3501
Coulomb’s Law
In 1785, Coulomb established the fundamental law of electric force between two stationary, charged particles. Experiments show that an electric force has the following properties:
(1) The force is inversely proportional to the square of separation, r2, between the two charged particles.
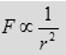
(2) The force is proportional to the product of charge q1 and the charge q2 on the particles.
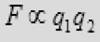
(3) The force is attractive if the charges are of opposite sign and repulsive if the charges have the same sign.
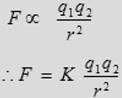
where K is the coulomb constant = 9 × 109 N.m2/C2.
The above equation is called Coulomb’s law, which is used to calculate the force between electric charges. In that equation F is measured in Newton (N), q is measured in unit of coulomb (C) and r in meter (m).
The constant K can be written as

where εο is known as the Permittivity constant of free space.
□ εο = 8.85 × 10-12 C2/N.m2
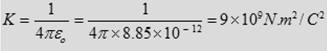
 الاكثر قراءة في الكهربائية
الاكثر قراءة في الكهربائية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


