
The effects of temperature on μeff
 المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
 المصدر:
INORGANIC CHEMISTRY
المصدر:
INORGANIC CHEMISTRY
 الجزء والصفحة:
2th ed p 583
الجزء والصفحة:
2th ed p 583
 22-2-2017
22-2-2017
 1180
1180
The effects of temperature on μeff
So far, we have ignored the effects of temperature on μeff. If a complex obeys the Curie Law (equation 1.2), then μeff is independent of temperature; this follows from a combination of equations 1.1 and 1.2.
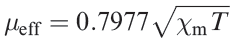 (1.1)
(1.1)
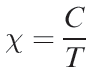 (1.2)
(1.2)
where C = Curie constant; T = temperature in K. However, the Curie Law is rarely obeyed and so it is essential to state the temperature at which a value of μeff has been measured. For second and third row d-block metal ions in particular, quoting only a room temperature value of μeff is usually meaningless; when spin–orbit coupling is large, μeffis highly dependent on T.
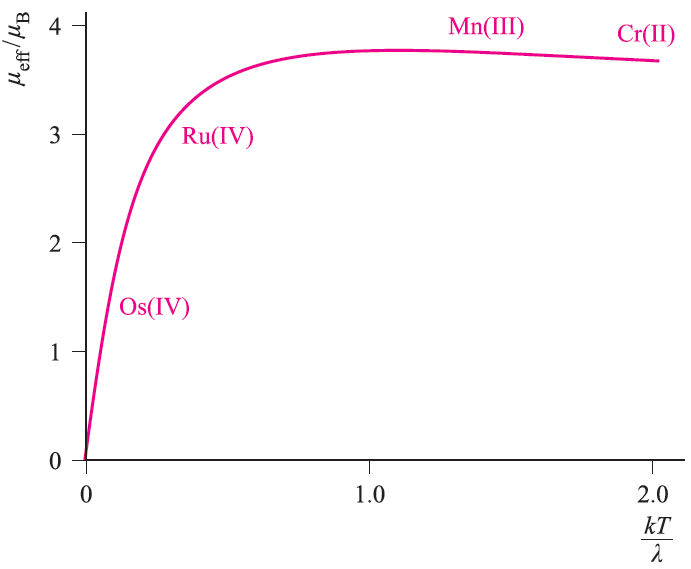
Fig. 1.1 Kotani plot for a t2g4 configuration; λ is the spin– orbit coupling constant. Typical values of μeff(298 K) for Cr(II), Mn(III), Ru(IV) and Os(IV) are indicated on the curve.
For a given electronic configuration, the influence of temperature on μeffcan be seen from a Kotani plot of μeff against kT/λ where k is the Boltzmann constant, T is the temperature in K, and λ is the spin–orbit coupling constant. Remember that λ is small for first row metal ions, is large for a second row metal ion, and is even larger for a third row ion. Figure 1.1 shows a Kotani plot for a t2g4 configuration; four points are indicated on the curve and correspond to typical values of μeff(298 K) for complexes of Cr(II) and Mn(III) from the first row, and Ru(IV) and Os(IV) from the second and third rows respectively. Points to note from these data are:
- since the points corresponding to μeff(298 K) for the first row metal ions lie on the near-horizontal part of the curve, changing the temperature has little effect on μeff;
- since the points relating to μeff(298 K) for the heavier metal ions lie on parts of the curve with steep gradients, μeff is sensitive to changes in temperature; this is especially true for Os(IV).
 الاكثر قراءة في كيمياء العناصر الانتقالية ومركباتها المعقدة
الاكثر قراءة في كيمياء العناصر الانتقالية ومركباتها المعقدة
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


