

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Polystyrene (PS)
المؤلف:
sami matar & Lewis. F. Hatch
المصدر:
Chemistry of PETROCHEMICAL PROCESSES
الجزء والصفحة:
p 334
24-9-2017
2513
Polystyrene (PS)
Polystyrene (PS) is the fourth big-volume thermoplastic. Styrene can be polymerized alone or copolymerized with other monomers. It can be polymerized by free radical initiators or using coordination catalysts. Recent work using group 4 metallocene combined with methylaluminoxane produce stereoregular polymer. When homogeneous titanium catalyst is used, the polymer was predominantly syndiotactic. The heterogeneous titanium catalyst gave predominantly the isotactic.
Copolymers with butadiene in a ratio of approximately 1:3 produces SBR, the most important synthetic rubber.
Copolymers of styrene-acrylonitrile (SAN) have higher tensile strength than styrene homopolymers. A copolymer of acrylonitrile, butadiene, and styrene (ABS) is an engineering plastic due to its better mechanical properties (discussed later in this chapter). Polystyrene is produced either by free radical initiators or by use of coordination catalysts.
Bulk, suspension, and emulsion techniques are used with free radical initiators, and the polymer is atactic. In a typical batch suspension process (Figure 1.1), styrene is suspended in water by agitation and use of a stabilizer.
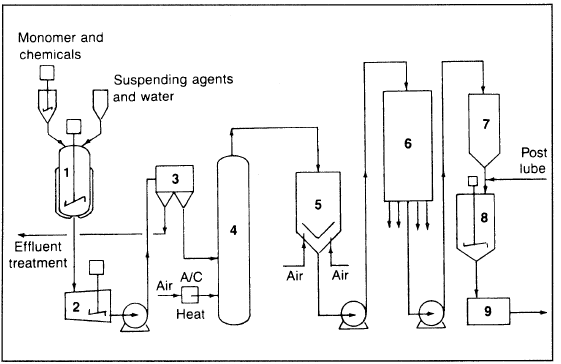
Figure 1.1. The Lummus Crest Inc. process for producing polystyrene: (1) reactor, (2) holding tank (Polystyrene beads and water), (3) centrifuge, (4) pneumatic drier, (5) conditioning tank, (6) screening of beads, (7,8) lubrication and blending, (9) shipping product.
The polymer forms beads. The bead/water slurry is separated by centrifugation, dried, and blended with additives.
 الاكثر قراءة في كيمياء البوليمرات
الاكثر قراءة في كيمياء البوليمرات
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















