
آخر المواضيع المضافة


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 26-11-2019
Date: 1-12-2019
Date: 1-8-2019
|
A Diels-Alder
A Diels-Alder approach to to tricyclic cedrene skeleton was reported by Breitholle et.al., (Can. J. Chem., 54, 1991 (1976) (Figure 8.8). Alkylation of cyclobutadiene to the requisite chain gave 8.8A after equilibration. The DA reaction proceeded in 36% yield to give a mixture of isomers. The ketone 8.8B did not undergo ring expantion with diazomethane. The ring expansion was finally achieved via the methylene amine 8.8C via diazotisation. The fact that two ring expansion products 8.8D and 8.8E were formed with 8.8D as the major product suggests that the amine 8.8F was the major isomer.
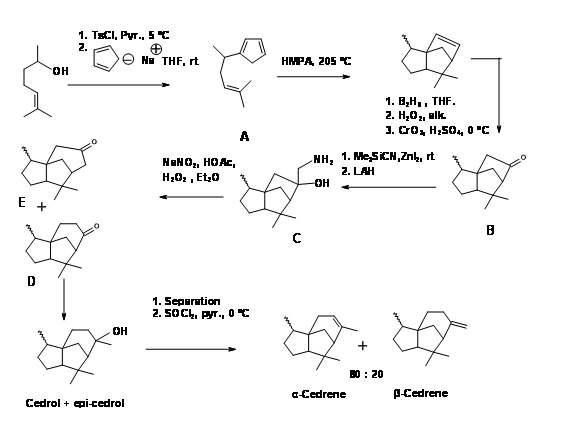
Figure 8.8



|
|
|
|
لصحة القلب والأمعاء.. 8 أطعمة لا غنى عنها
|
|
|
|
|
|
|
حل سحري لخلايا البيروفسكايت الشمسية.. يرفع كفاءتها إلى 26%
|
|
|
|
|
|
|
جامعة الكفيل تحتفي بذكرى ولادة الإمام محمد الجواد (عليه السلام)
|
|
|