
آخر المواضيع المضافة


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 1-2-2018
Date: 22-11-2018
Date: 22-10-2018
|
Phosphorus(III) oxide is a white solid, melting at 24°C and boiling at 173°C. The structure of its molecule is best worked out starting from a P4 molecule which is a little tetrahedron.

Pull this apart so that you can see the bonds . . .
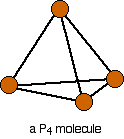
. . . and then replace the bonds by new bonds linking the phosphorus atoms via oxygen atoms. These will be in a V-shape (rather like in water), but you probably wouldn't be penalised if you drew them on a straight line between the phosphorus atoms in an exam.
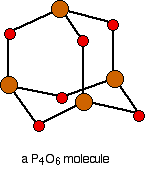
The phosphorus is using only three of its outer electrons (the 3 unpaired p electrons) to form bonds with the oxygens.



|
|
|
|
دراسة تحدد أفضل 4 وجبات صحية.. وأخطرها
|
|
|
|
|
|
|
العتبة العباسية تستعدّ لتكريم عددٍ من الطالبات المرتديات للعباءة الزينبية في جامعات كركوك
|
|
|