


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 25-7-2019
Date: 4-10-2020
Date: 6-6-2016
|
The products of bond breaking, shown above, are not stable in the usual sense, and cannot be isolated for prolonged study. Such species are referred to as reactive intermediates, and are believed to be transient intermediates in many reactions. The general structures and names of four such intermediates are given below.
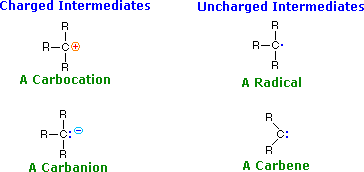
A pair of widely used terms, related to the Lewis acid-base notation, should also be introduced here.
Using these definitions, it is clear that carbocations ( called carbonium ions in the older literature ) are electrophiles and carbanions are nucleophiles. Carbenes have only a valence shell sextet of electrons and are therefore electron deficient. In this sense they are electrophiles, but the non-bonding electron pair also gives carbenes nucleophilic character. As a rule, the electrophilic character dominates carbene reactivity. Carbon radicals have only seven valence electrons, and may be considered electron deficient; however, they do not in general bond to nucleophilic electron pairs, so their chemistry exhibits unique differences from that of conventional electrophiles. Radical intermediates are often called free radicals.
The importance of electrophile / nucleophile terminology comes from the fact that many organic reactions involve at some stage the bonding of a nucleophile to an electrophile, a process that generally leads to a stable intermediate or product. Reactions of this kind are sometimes called ionic reactions, since ionic reactants or products are often involved. Some common examples of ionic reactions and their mechanisms may be examined below.
The shapes ideally assumed by these intermediates becomes important when considering the stereochemistry of reactions in which they play a role. A simple tetravalent compound like methane, CH4, has a tetrahedral configuration. Carbocations have only three bonds to the charge bearing carbon, so it adopts a planar trigonal configuration. Carbanions are pyramidal in shape ( tetrahedral if the electron pair is viewed as a substituent ), but these species invert rapidly at room temperature, passing through a higher energy planar form in which the electron pair occupies a p-orbital. Radicals are intermediate in configuration, the energy difference between pyramidal and planar forms being very small. Since three points determine a plane, the shape of carbenes must be planar; however, the valence electron distribution varies.



|
|
|
|
دراسة: حفنة من الجوز يوميا تحميك من سرطان القولون
|
|
|
|
|
|
|
تنشيط أول مفاعل ملح منصهر يستعمل الثوريوم في العالم.. سباق "الأرنب والسلحفاة"
|
|
|
|
|
|
|
الطلبة المشاركون: مسابقة فنِّ الخطابة تمثل فرصة للتنافس الإبداعي وتنمية المهارات
|
|
|