


 الفيزياء الكلاسيكية
الفيزياء الكلاسيكية
 الكهربائية والمغناطيسية
الكهربائية والمغناطيسية
 علم البصريات
علم البصريات
 الفيزياء الحديثة
الفيزياء الحديثة
 النظرية النسبية
النظرية النسبية
 الفيزياء النووية
الفيزياء النووية
 فيزياء الحالة الصلبة
فيزياء الحالة الصلبة
 الليزر
الليزر
 علم الفلك
علم الفلك
 المجموعة الشمسية
المجموعة الشمسية
 الطاقة البديلة
الطاقة البديلة
 الفيزياء والعلوم الأخرى
الفيزياء والعلوم الأخرى
 مواضيع عامة في الفيزياء
مواضيع عامة في الفيزياء|
Read More
Date: 13-2-2021
Date: 19-2-2021
Date: 16-2-2021
|
The magnetic periodic table
Table A displays the magnetic properties of the elements, distinguishing those that are paramagnetic, diamagnetic, ferromagnetic or antiferromagnetic at room temperature, and those that order magnetically at some lower temperature. Only sixteen elements have a magnetically ordered ground state, and all but oxygen belong to the 3d or 4f transition series. Besides iron, cobalt and nickel, only gadolinium can be ferromagnetic at room temperature, but that depends on the weather! The Curie temperature of gadolinium is just 292 K.
Many other elements become superconducting at low enough temperature. The remainder are neither magnetic nor superconducting. No element manages to be both at the same time.
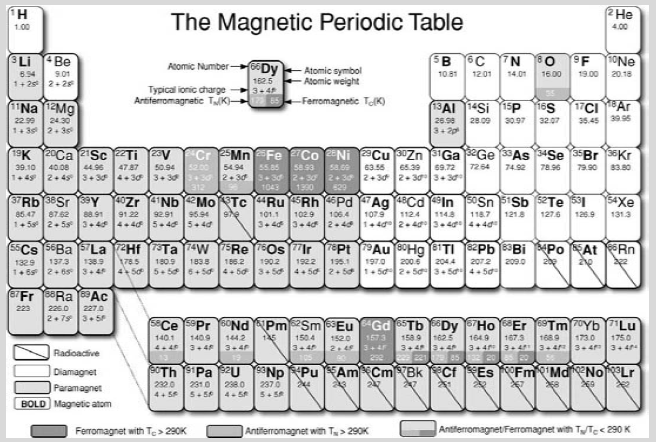
Table A The magnetic periodic table. Diamagnetic elements are uncoloured, paramagnets are pale grey, ferromagnets are dark grey, antiferromagnets are mid grey, and the Curie or Ne´ el temperatures are marked. Common paramagnetic ions are indicated. Elements which bear a magnetic moment as isolated atoms are marked in bold type



|
|
|
|
لصحة القلب والأمعاء.. 8 أطعمة لا غنى عنها
|
|
|
|
|
|
|
حل سحري لخلايا البيروفسكايت الشمسية.. يرفع كفاءتها إلى 26%
|
|
|
|
|
|
|
جامعة الكفيل تحتفي بذكرى ولادة الإمام محمد الجواد (عليه السلام)
|
|
|