


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 11-7-2016
Date: 25-7-2019
Date: 4-10-2020
|
Hydrogen addition to multiple bonds is catalyzed by certain complex metal salts in solution. This may be described as homogeneous catalysis and, compared to heterogeneous catalysis, is a relatively new development in the area of hydrogenation reactions. Rhodium and ruthenium salts appear to be generally useful catalysts:
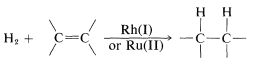
At present, homogeneous catalysis for routine hydrogenation reactions offers little advantage over the convenience and simplicity of heterogeneous catalysis. Suprafacial addition of hydrogen is observed with both types of catalytic systems. However, greater selectivity can be achieved with homogeneous catalysts because they appear to be more sensitive to steric hindrance and are less likely to cause rearrangement, dissociation, and hydrogenation of other bonds (e.g., −NO2 and 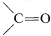 ).
).
The most thoroughly investigated homogeneous hydrogenation catalyst is the four-coordinated rhodium complex Rh[(C6H5)3P]3Cl. This catalyst is called Wilkinson's catalyst after its discoverer, G. Wilkinson. In 1973, the Nobel Prize in chemistry was awarded jointly to Wilkinson and E. O. H. Fischer for their respective contributions to the field of organometallic chemistry. As you will see in this and later chapters, compounds with carbon-metal bonds (organometallic compounds) are extremely useful reagents, reactive intermediates, or catalysts in organic reactions.



|
|
|
|
دراسة: حفنة من الجوز يوميا تحميك من سرطان القولون
|
|
|
|
|
|
|
تنشيط أول مفاعل ملح منصهر يستعمل الثوريوم في العالم.. سباق "الأرنب والسلحفاة"
|
|
|
|
|
|
|
الطلبة المشاركون: مسابقة فنِّ الخطابة تمثل فرصة للتنافس الإبداعي وتنمية المهارات
|
|
|