
Natural Killer Cells
 المؤلف:
Abbas, A. K., Lichtman, A. H., & Pillai, S
المؤلف:
Abbas, A. K., Lichtman, A. H., & Pillai, S
 المصدر:
Basic Immunology : Function and disorders of immune system
المصدر:
Basic Immunology : Function and disorders of immune system
 الجزء والصفحة:
6th ed , page 36-38
الجزء والصفحة:
6th ed , page 36-38
 2025-01-09
2025-01-09
 999
999
NK cells recognize infected and stressed cells and respond by killing these cells and by secreting the macrophage-activating cytokine IFN-γ (Fig. 2.12). NK cells are developmentally related to group 1 ILCs and make up approximately 10% of the cells with lymphocyte morphology in the blood and peripheral lymphoid organs. NK cells contain cytoplasmic granules and express some unique surface proteins but do not express immunoglobulins or T cell receptors, the antigen receptors of B and T lymphocytes, respectively. On activation by infected cells, NK cells empty the contents of their cytoplasmic granules into the extra cellular space at the point of contact with the infected cell. The granule proteins then enter infected cells and activate enzymes that induce apoptosis. The cytotoxic mechanisms of NK cells, which are the same as the mechanisms used by cytotoxic T lymphocytes (CTLs), result in the death of infected cells. Thus, as with CTLs, NK cells function to eliminate cellular reservoirs of infection and eradicate infections by obligate intracellular microbes, such as viruses. In addition, NK cells may contribute to the destruction of tumors.
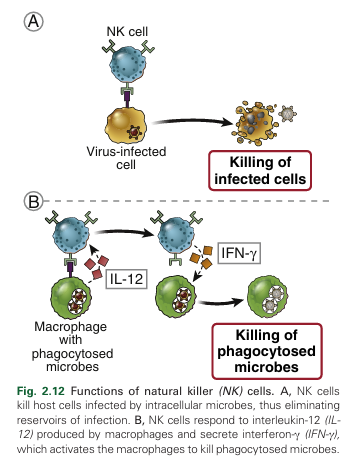
Activated NK cells also synthesize and secrete the cytokine interferon-γ (IFN-γ), which activates macro phages to become more effective at killing phagocytosed microbes. Cytokines secreted by macrophages and dendritic cells that have encountered microbes enhance the ability of NK cells to protect against infections. Three of these NK cell–activating cytokines are interleukin-15 (IL-15), type I IFNs, and interleukin-12 (IL-12). IL-15 is important for the development and maturation of NK cells, and type I IFNs and IL-12 enhance the killing functions of NK cells. Thus, NK cells and macrophages are examples of two cell types that function cooperatively to eliminate intracellular microbes: macrophages ingest microbes and produce IL-12, IL-12 activates NK cells to secrete IFN-γ, and IFN-γ in turn activates the macrophages to kill the ingested microbes. As discussed in Chapter 6, essentially the same sequence of reactions involving macrophages and T lymphocytes is central to the cell-mediated arm of adaptive immunity.
The activation of NK cells is determined by a balance between engagement of activating and inhibitory receptors (Fig. 2.13).
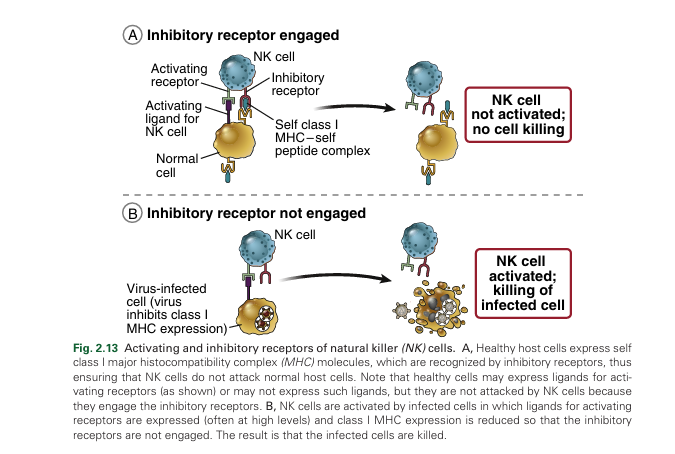
The activating receptors recognize cell surface molecules typically expressed on cells infected with viruses and intracellular bacteria , some cancer cells, and cells stressed by DNA damage. These receptors enable NK cells to eliminate cells infected with intracellular microbes, as well as irreparably injured cells and tumor cells. One of the well-defined activating receptors of NK cells is called NKG2D; it recognizes molecules that resemble class I major histocompatibility complex (MHC) proteins and are expressed in response to many types of cellular stress. Another activating receptor, called CD16, is specific for immunoglobulin G (IgG) antibodies bound to cells. The recognition of antibody-coated cells results in killing of these cells, a phenomenon called antibody-de pendent cellular cytotoxicity (ADCC). NK cells are the principal mediators of ADCC.. Activating receptors on NK cells have signaling subunits that contain immunoreceptor tyrosine-based activation motifs (ITAMs) in their cytoplasmic tails. ITAMs, which also are present in subunits of lymphocyte antigen receptor–associated signaling molecules, become phosphorylated on tyrosine residues when the receptors recognize their activating ligands. T he phosphorylated ITAMs bind and promote the activation of cytosolic protein tyrosine kinases, and these enzymes phosphorylate, and thereby activate, other substrates in several different downstream signal transduction pathways, eventually leading to cytotoxic granule exocytosis and production of IFN-γ. T he inhibitory receptors of NK cells block signaling by activating receptors and are specific for self class I MHC molecules, which are expressed on all healthy nucleated cells. Therefore, class I MHC expression protects healthy cells from destruction by NK cells. Two major families of NK cell inhibitory receptors in humans are the killer cell immunoglobulin-like receptors (KIRs), so called because they share structural homology with Ig molecules , and receptors consisting of a protein called CD94 and a lectin subunit called NKG2. Both families of inhibitory receptors contain in their cytoplasmic tails structural motifs called immunoreceptor tyrosine-based inhibitory motifs (ITIMs), which become phosphorylated on tyrosine residues when the receptors bind class I MHC molecules. The phosphorylated ITIMs bind and promote activation of cytosolic protein tyrosine phosphatases. These enzymes remove phosphate groups from the tyrosine residues of various signaling molecules, thereby counteracting the function of the ITAMs and blocking the activation of NK cells through activating receptors. Therefore, when the inhibitory receptors of NK cells encounter self MHC molecules on normal host cells, the NK cells are shut off (see Fig. 2.13). Many viruses have developed mechanisms to block expression of class I molecules in infected cells, which allows them to evade killing by virus-specific CD8+ CTLs. When this happens, the NK cell inhibitory receptors are not engaged, and if the virus induces expression of activating ligands at the same time, the NK cells become activated and eliminate the virus-infected cells. T he role of NK cells and CTLs in defense illustrates how hosts and microbes are engaged in a constant struggle for survival. The host uses CTLs to recognize MHC-dis played viral antigens, viruses inhibit MHC expression to evade killing of the infected cells by CTLs, and NK cells can compensate for the defective CTL response because the NK cells are more effective in the absence of MHC molecules. The winner of this struggle, the host or the microbe, determines the outcome of the infection. The same principles may apply to the functions of NK cells in eradication of tumors, many of which also attempt to escape from CTL-mediated killing by reducing expression of class I MHC molecules .
 الاكثر قراءة في المناعة
الاكثر قراءة في المناعة
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


