


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 12-2-2016
Date: 12-2-2016
Date: 12-2-2016
|
DEHYDROGENATION
Dehydrogenation is a reaction that results in the removal of hydrogen from an organic compound or compounds, as in the dehydrogenation of ethane to ethylene:
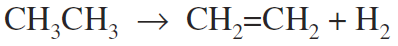
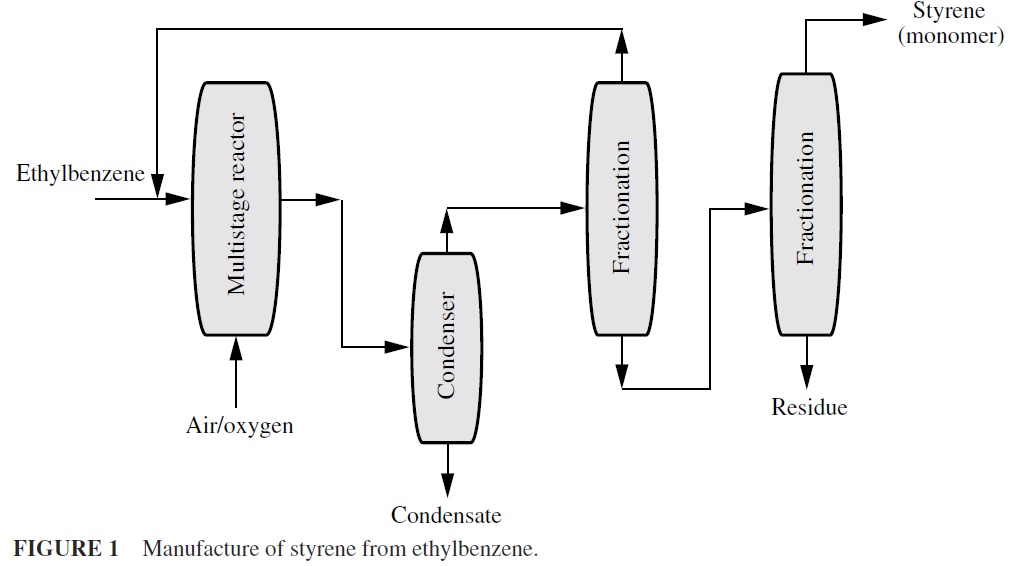
This process is brought about in several ways. The most common method is to heat hydrocarbons to high temperature, as in thermal cracking, that causes some dehydrogenation, indicated by the presence of unsaturated compounds and free hydrogen. In the chemical process industries, nickel, cobalt, platinum, palladium, and mixtures containing potassium, chromium, copper, aluminum, and other metals are used in very large-scale dehydrogenation processe Styrene is produced from ethylbenzene by dehydrogenation (Fig. 1). Many lower molecular weight aliphatic ketones are made by dehydration of alcohols. Acetone, methyl ethyl ketone, and cyclohexanone can be made in this fashion.
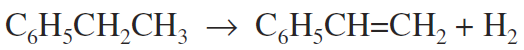
Acetone is the ketone used in largest quantity and is produced as a by-product of the manufacture of phenol via cumene. Manufacture from iso-propanol is by the reaction:

This reaction takes place at 350 oC and 200 kPa with copper or zinc acetate as the catalyst; conversion is 85 to 90 percent. Purification by distillation follows. The dehydrogenation of n-paraffins yields detergent alkylates and n-olefins. The catalytic use of rhenium for selective dehydrogenation has increased in recent years since dehydrogenation is one of the most commonly practiced of the chemical unit processes.



|
|
|
|
لصحة القلب والأمعاء.. 8 أطعمة لا غنى عنها
|
|
|
|
|
|
|
حل سحري لخلايا البيروفسكايت الشمسية.. يرفع كفاءتها إلى 26%
|
|
|
|
|
|
|
جامعة الكفيل تحتفي بذكرى ولادة الإمام محمد الجواد (عليه السلام)
|
|
|