


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 24-12-2018
Date: 4-12-2018
Date: 13-3-2019
|
Electron affinities
The first electron affinity (EA1) is minus the internal energy change (equation 1.1) for the gain of an electron by a gaseous atom (equation 1.2). The second electron affinity of atom Y is defined for process 1.3. Each reaction occurs in the gas phase.
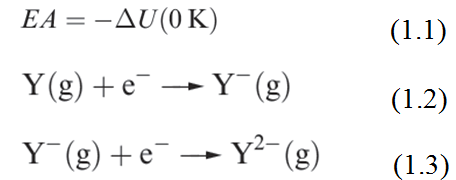
As we saw for ionization energies, it is convenient to define an enthalpy change, ΔEAH, associated with each of the reactions 1. 1 and 1. 2. We approximate ΔEAH(298K) to ΔEAU(0K). Selected values of these enthalpy changes are given in Table 1.1.
Table 1.1 Approximate enthalpy changes ΔEAH(298 K) associated with the attachment of an electron to an atom or anion.
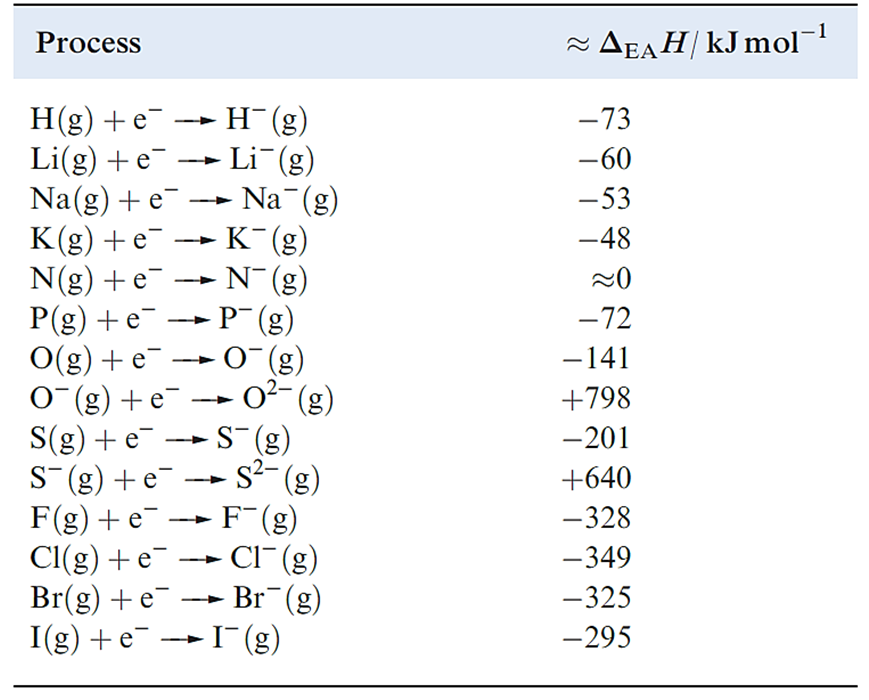
The attachment of an electron to an atom is usually exothermic. Two electrostatic forces oppose one another: the repulsion between the valence shell electrons and the additional electron, and the attraction between the nucleus and the incoming electron. In contrast, repulsive interactions are dominant when an electron is added to an anion and the process is endothermic (Table 1.1).



|
|
|
|
دراسة تحدد أفضل 4 وجبات صحية.. وأخطرها
|
|
|
|
|
|
|
العتبة العباسية تستعدّ لتكريم عددٍ من الطالبات المرتديات للعباءة الزينبية في جامعات كركوك
|
|
|