


 الفيزياء الكلاسيكية
الفيزياء الكلاسيكية
 الكهربائية والمغناطيسية
الكهربائية والمغناطيسية
 علم البصريات
علم البصريات
 الفيزياء الحديثة
الفيزياء الحديثة
 النظرية النسبية
النظرية النسبية
 الفيزياء النووية
الفيزياء النووية
 فيزياء الحالة الصلبة
فيزياء الحالة الصلبة
 الليزر
الليزر
 علم الفلك
علم الفلك
 المجموعة الشمسية
المجموعة الشمسية
 الطاقة البديلة
الطاقة البديلة
 الفيزياء والعلوم الأخرى
الفيزياء والعلوم الأخرى
 مواضيع عامة في الفيزياء
مواضيع عامة في الفيزياء|
Read More
Date: 11-8-2016
Date: 13-7-2016
Date: 18-8-2016
|
Gas Mixture Condensation
A mixture of mN = 100 g of nitrogen and some oxygen is isothermally compressed at T = 77.4 K. The result of this experiment is plotted as the pressure dependence of the mixture versus volume in arbitrary units (see Figure 1.1). Find the mass of oxygen and the oxygen saturation vapor pressure at this temperature.

Figure 1.1
Hint: T = 77.4 K is the boiling temperature of liquid nitrogen at atmospheric pressure. Oxygen boils at a higher temperature.
SOLUTION
Consider three parts of the plot (see Figure 1.2). At V > V2 there is a regular gas mixture (no condensation). At V1 < V < V2, one of the gases is condensing; let us assume for now it is oxygen (it happens to be true). At V < V1 they are both condensing, and there is no pressure change. Let us write
 (1)
(1)
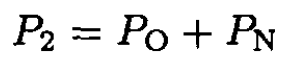 (2)
(2)
Here PN is the partial nitrogen pressure at (V2, P2), PO is the saturation vapor pressure of oxygen, and PA is the saturated vapor pressure of nitrogen (1 atm) at T = 77.4 K. Between V2 and V1, nitrogen is a gas, and since the temperature is constant,
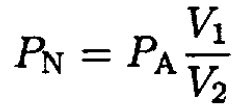 (3)
(3)
Using (3) and dividing (1) by (2), we have
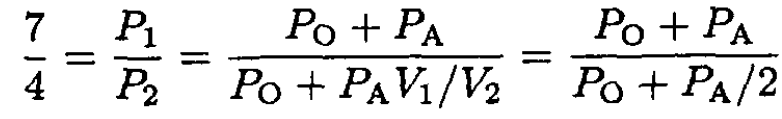 (4)
(4)
yielding

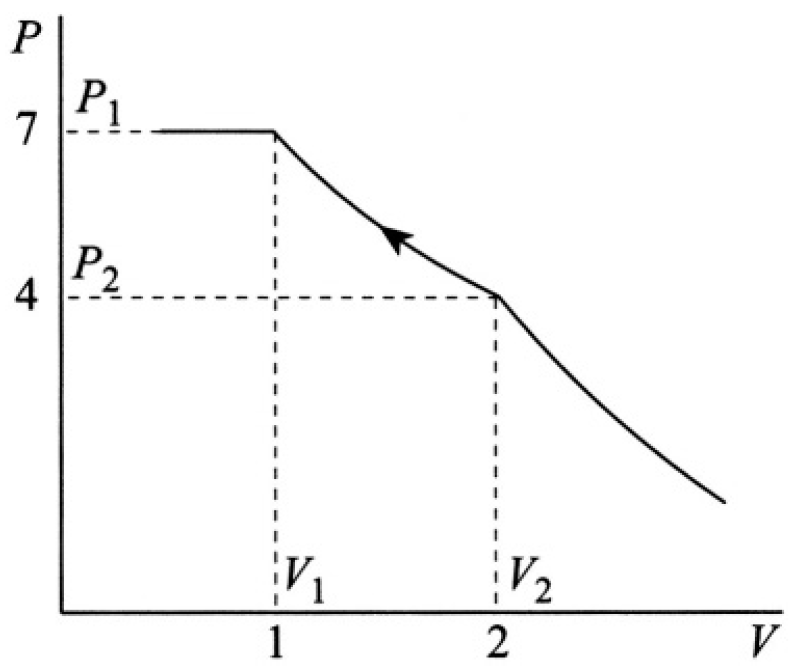
Figure 1.2
Had we assumed that oxygen is condensing at (V1, P1) we would get 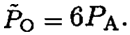 This contradicts the fact that oxygen boils at a higher temperature. The saturated vapor pressure at T = 77.4 K should be less than PA. To find the oxygen mass, we use the ideal gas law at (V2, P2) where the oxygen is just starting to condense (i.e., its pressure is PO and it is all gas). So
This contradicts the fact that oxygen boils at a higher temperature. The saturated vapor pressure at T = 77.4 K should be less than PA. To find the oxygen mass, we use the ideal gas law at (V2, P2) where the oxygen is just starting to condense (i.e., its pressure is PO and it is all gas). So
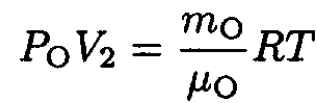 (5)
(5)
where μO is the oxygen molar mass. For nitrogen a similar equation can be written for (V1, P1):
 (6)
(6)
where μN is the molar mass of nitrogen. Dividing (5) by (6), we obtain
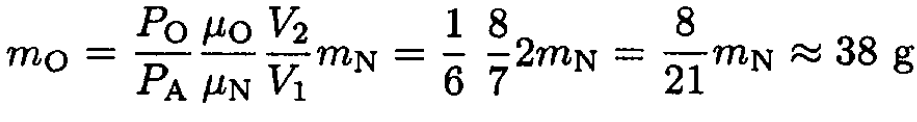



|
|
|
|
دراسة: حفنة من الجوز يوميا تحميك من سرطان القولون
|
|
|
|
|
|
|
تنشيط أول مفاعل ملح منصهر يستعمل الثوريوم في العالم.. سباق "الأرنب والسلحفاة"
|
|
|
|
|
|
|
لتعزيز التواصل مع الزائرات الأجنبيات : العتبة العلويّة المقدّسة تُطلق دورة لتعليم اللغة الإنجليزية لخادمات القسم النسويّ
|
|
|