
Surface Contamination
 المؤلف:
Sidney B. Cahn, Gerald D. Mahan And Boris E. Nadgorny
المؤلف:
Sidney B. Cahn, Gerald D. Mahan And Boris E. Nadgorny
 المصدر:
A GUIDE TO PHYSICS PROBLEMS
المصدر:
A GUIDE TO PHYSICS PROBLEMS
 الجزء والصفحة:
part 2 , p 10
الجزء والصفحة:
part 2 , p 10
 4-9-2016
4-9-2016
 1270
1270
Surface Contamination
A surface scientist wishes to keep an exposed surface “clean” (≤ 0.05 adsorbed monolayer) for an experiment lasting for times t ≥ h at a temperature T = 300 K. Estimate the needed data and calculate a value for the required background pressure in the apparatus if each incident molecule sticks to the surface.
SOLUTION
The number of molecules striking a unit area of the surface N during the time of the experiment is given by
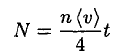 (1)
(1)
For an estimate we can assume that the adsorbed molecules are closely packed and that the number of adsorption sites on a surface of area A is just
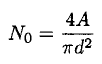 (2)
(2)
where d is the average diameter of the adsorbed atoms, and we take d ~ 3A.The total number of adsorption sites may actually be smaller (these data can be obtained from the time to create one monolayer at lower pressure). We may write
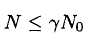
or, for 1 m2 of surface,
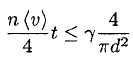
Using the average velocity at T = 300 K gives
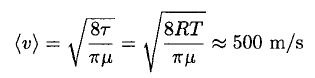 (3)
(3)
and
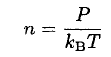 (4)
(4)
Thus,
 (5)
(5)
So, we will have to maintain a pressure better than 10-11 Torr, which can be quite a technical challenge. In fact, at such low pressures the residual gas composition is somewhat different from room air, since it may be more difficult to pump gases such as H2 and He. Therefore, (3) and (5) are only order-of-magnitude estimates.
 الاكثر قراءة في مواضيع اخرى
الاكثر قراءة في مواضيع اخرى
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


