علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Putting the quantum numbers together
المؤلف:
John T. Moore, EdD
المصدر:
Chemistry Essentials For Dummies
الجزء والصفحة:
P25
26-12-2016
2511
Putting the quantum numbers together
Table 1.1 summarizes the quantum numbers available for the first two energy levels.
Table 1.1 Quantum Numbers for the First Two Energy Levels
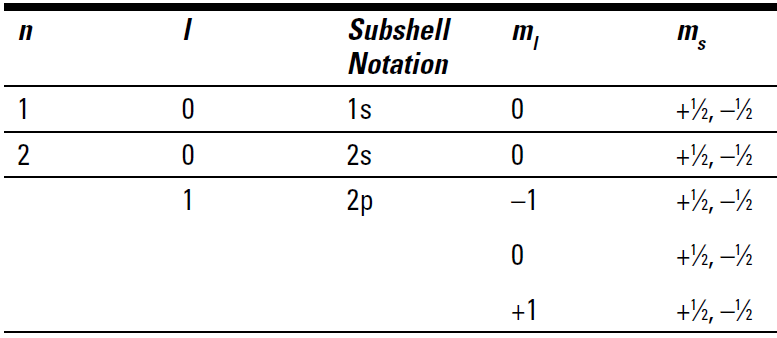
Table 1.1 shows that in energy level 1 (n = 1), there’s only an s orbital. There’s no p orbital because an l value of 1 (p orbital) is not allowed. And notice that there can be only two electrons in that 1s orbital (ms of +1⁄2 and –1⁄2). In fact, there can be only two electrons in any s orbital, whether it’s 1s or 5s.
Each time you move higher in a major energy level, you add another orbital type. So when you move from energy level 1 to energy level 2 (n = 2), there can be both s and p orbitals. If you write out the quantum numbers for energy level 3, you see s, p, and d orbitals.
Notice also that there are three subshells (ml ) for the 2p orbital and that each holds a maximum of two electrons. The three 2p subshells can hold a maximum of six electrons.
There’s an energy difference in the major energy levels (energy level 2 is higher in energy than energy level 1), but there’s also a difference in the energies of the different orbitals within an energy level. At energy level 2, both s and p orbitals are present. But the 2s is lower in energy than the 2p. The three subshells of the 2p orbital have the same energy. Likewise, the five subshells of the d orbitals have the same energy.
 الاكثر قراءة في مقالات متنوعة في علم الكيمياء
الاكثر قراءة في مقالات متنوعة في علم الكيمياء
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)