

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Coordination number 7
المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
المصدر:
INORGANIC CHEMISTRY
الجزء والصفحة:
2th ed p 545
23-2-2017
1363
Coordination number 7
High coordination numbers (≥7) are observed most frequently for ions of the early second and third row d-block metals and for the lanthanoids and actinoids, i.e. rcation must be relatively large. Figure 1.1a shows the arrangement of the donor atoms for the three idealized 7- coordinate structures; in the capped trigonal prism, the ‘cap’ is over one of the square faces of the prism. In reality, there is much distortion from these idealized structures, and this is readily apparent for the example of a capped octahedral complex shown in Figure 1.1b. The anions in [Li(OEt2)][MoMe7]- and [Li(OEt2)][WMe7]- are further examples of capped octahedral structures. A problem in the chemical literature is that the distortions may lead to ambiguity in the way in which a given structure is described. Among binary metal halides and pseudo-halides, 7-coordinate structures are exemplified by the pentagonal bipyramidal ions [V(CN)7]4- (d2) and [NbF7]3- (d1). In the ammonium salt, [ZrF7]3- (d0) is pentagonal bipyramidal, but in the guanidinium salt, it has a monocapped trigonal prismatic structure (Figure 1.1c).
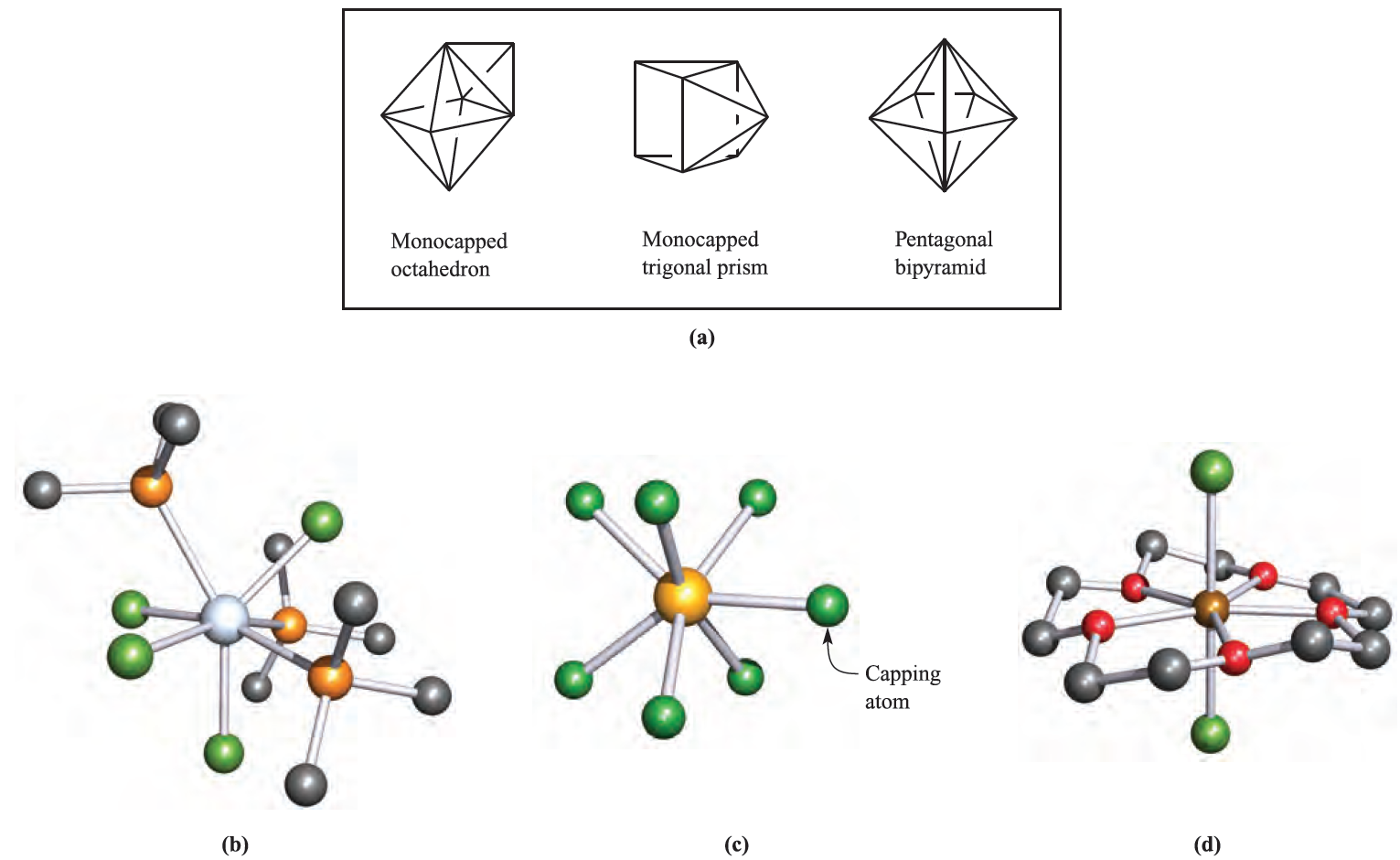
Fig. 1.1 (a) The coordination spheres defined by the donor atoms in idealized 7-coordinate structures. Examples of
7-coordinate complexes (X-ray diffraction data): (b) the capped octahedral structure of [TaCl4(PMe3)3] [F.A. Cotton et al. (1984) Inorg. Chem., vol. 23, p. 4046], (c) the capped trigonal prismatic [ZrF7]3- in the guanidinium salt [A.V. Gerasimenko et al. (1985) Koord. Khim., vol. 11, p. 566], and (d) the pentagonal bipyramidal cation in [ScCl2(15-crown-5)]2[CuCl4] with the crown ether occupying the equatorial plane [N.R. Strel’tsova et al. (1992) Zh. Neorg. Khim., vol. 37, p. 1822]. Hydrogen atoms have been omitted for clarity; colour code: Ta, silver; Cl, green; P, orange; Zr, yellow; F, green; Sc, brown; C, grey; O, red.
 الاكثر قراءة في كيمياء العناصر الانتقالية ومركباتها المعقدة
الاكثر قراءة في كيمياء العناصر الانتقالية ومركباتها المعقدة
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















