


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 13-11-2020
Date: 29-6-2020
Date: 6-2-2018
|
Dithionic acid, H2S2O6
Dithionic acid is another sulfur oxoacid that is only known in aqueous solution (in which it behaves as a strong acid) or in the form of salts containing the dithionate, [S2O6]2-, ion. Such salts can be isolated as crystalline solids and Figure 15.17a shows the presence of a long S_S bond; the anion possesses a staggered conformation in the solid state. The dithionate ion can be prepared by controlled oxidation of [SO3]2- (equations 1.1 and 1.2), but not by the reduction of [SO4]2- (equation 1.3).
The [S2O6]2- can be isolated as the soluble salt BaS2O6, which is easily converted into salts of other cations.
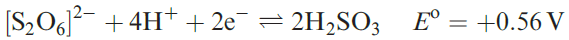 (1.1)
(1.1)
 (1.2)
(1.2)
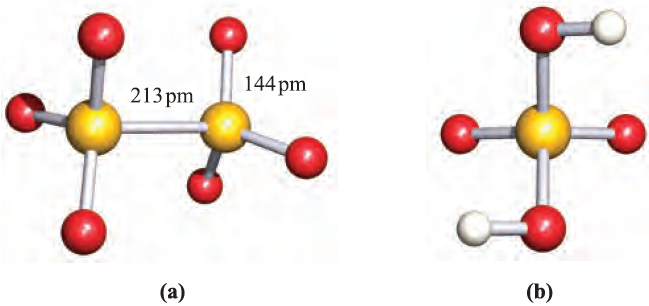
Fig. 1.1(a) The structure of [S2O6]2- showing the staggered conformation; from the salt [Zn{H2NNHC)O(Me}3][S2O6]. 2.5H2O [I.A. Krol et al. (1981) Koord. Khim., vol. 7, p. 800]; (b) the C2 structure of gas-phase H2SO4. Colour code: S, yellow; O, red; H, white.
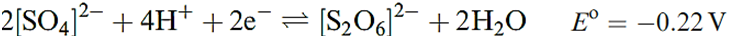 (1.3)
(1.3)
The [S2O6]2_ ion is not easily oxidized or reduced, but in acidic solution it slowly decomposes according to equation 1.4, consistent with there being a weak S_S bond.
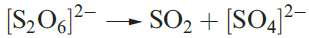 (1.4)
(1.4)



|
|
|
|
لصحة القلب والأمعاء.. 8 أطعمة لا غنى عنها
|
|
|
|
|
|
|
حل سحري لخلايا البيروفسكايت الشمسية.. يرفع كفاءتها إلى 26%
|
|
|
|
|
|
|
جامعة الكفيل تحتفي بذكرى ولادة الإمام محمد الجواد (عليه السلام)
|
|
|