


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 14-7-2019
Date: 29-11-2019
Date: 30-10-2019
|
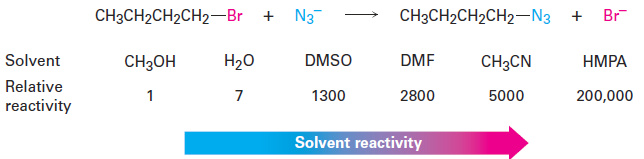
SN2 reactions-The Solvent
The rates of SN2 reactions are strongly affected by the solvent. Protic solvents— those that contain an - OH or - NH group—are generally the worst for SN2 reactions, while polar aprotic solvents, which are polar but don’t have an - OH
or - NH group, are the best. Protic solvents, such as methanol and ethanol, slow down SN2 reactions by solvation of the reactant nucleophile. The solvent molecules hydrogenbond to the nucleophile and form a cage around it, thereby lowering its energy and reactivity.

In contrast with protic solvents—which decrease the rates of SN2 reactions by lowering the ground-state energy of the nucleophile—polar aprotic solvents increase the rates of SN2 reactions by raising the ground-state energy of the nucleophile. Acetonitrile (CH3CN), dimethylformamide [(CH3)2NCHO, abbreviated DMF-, dimethyl sulfoxide [(CH3)2SO, abbreviated DMSO-, and hexamethylphosphoramide {[(CH3)2N-3PO, abbreviated HMPA} are particularly useful. These solvents can dissolve many salts because of their high polarity, but they tend to solvate metal cations rather than nucleophilic anions. As a result, the bare, unsolvated anions have a greater nucleophilicity and SN2 reactions take place at correspondingly increased rates. For instance, a rate increase of 200,000 has been observed on changing from methanol to HMPA for the reaction of azide ion with 1-bromobutane.



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|