


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 5-3-2017
Date: 20-12-2018
Date: 10-12-2018
|
Neutral hydrides of group 13 elements
With three valence electrons, each group 13 element might be expected to form a hydride MH3. Although the existence of BH3 has been established in the gas phase, its propensity to dimerize means that B2H6 (diborane(6)) is, in practice, the simplest hydride of boron.
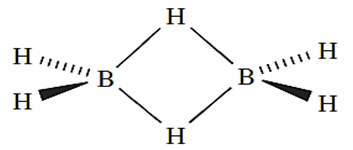
We have already discussed the structure of and bonding in B2H6 the reader is reminded of the presence of 3c-2e (delocalized, 3-centre 2-electron) B_H_B interactions.
Diborane(6) is an important reagent in synthetic organic chemistry, and reaction showen in below is one convenient laboratory preparation..
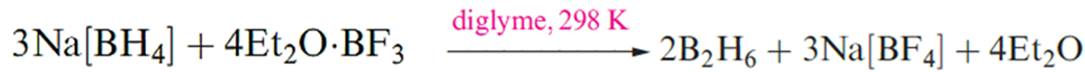
Although this reaction is standard procedure for the preparation of B2H6, it is not without problems. For example, the reaction temperature must be carefully controlled because the solubility of Na[BH4] in diglyme varies significantly with temperature. Secondly, the solvent cannot easily be recycled. Reaction that showen in below , which uses a triglyme adduct of BF3 as precursor, produces B2H6 quantitatively and is an improvement on the traditional reaction showen in above . Reaction below can be applied to large-scale syntheses, and the triglyme solvent can be recycled. Tetraglyme can be used in place of triglyme in below reaction .
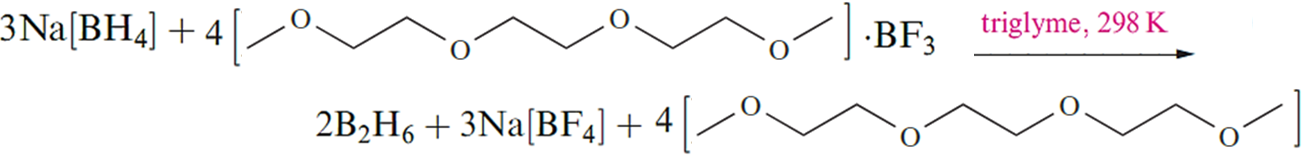
Reaction 12.10 is the basis for an industrial synthesis of B2H6.

Diborane(6) is a colourless gas (bp 180.5 K) which is rapidly decomposed by wate). Like other boron hydrides B2H6 has a small positive value of Δf Ho (36 kJ mol_1); mixtures with air or O2 are liable to inflame or explode.
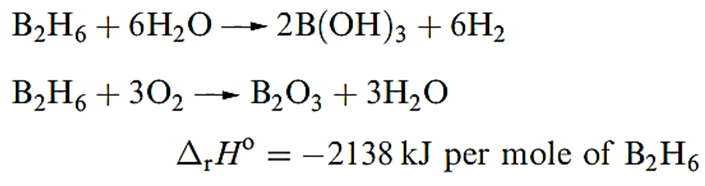



|
|
|
|
دراسة تحدد أفضل 4 وجبات صحية.. وأخطرها
|
|
|
|
|
|
|
جامعة الكفيل تحتفي بذكرى ولادة الإمام محمد الجواد (عليه السلام)
|
|
|