
The inorganic composition of cells
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص723-724
الجزء والصفحة:
ص723-724
 2025-10-22
2025-10-22
 54
54
The inorganic composition of cells
Key points: The major biological elements are oxygen, hydrogen, carbon, nitrogen, phosphorus, sulfur, sodium, magnesium, calcium, and potassium. The trace elements include many d metals, as well as selenium, iodine, silicon, and boron. Table 27.1 lists many of the elements known to be used in living systems, although not ne cessarily by higher life forms. All the second- and third-period elements except Be, Al, and the noble gases are used, as are most of the 3d elements, whereas Cd, Br, I, Mo, and W are the only heavier elements so far confirmed to have a biological function. Several others, such as Li, Ga, Tc, Ru, Gd, Pt, and Au, have important and increasingly well-understood applications in medicine. The biologically essential elements can be classified as either ‘major’or ‘trace’. Although a good idea of the biological abundances of different elements is given in Table 27.1, the levels vary considerably among organisms and different components of organisms. For example, Ca has little role in microorganisms but is abundant in higher life forms, whereas the use of Co by higher organisms depends on it being incorporated into a special co factor (cobalamin) by microorganisms. There is probably a universal requirement for K, Mg, Fe, and Mo. Vanadium is used by lower animals and plants as well as some bacteria. Nickel is essential for most microorganisms, and is used by plants, but there is no evidence for any direct role in animals. Nature’s use of different elements is largely based on their availability. For example, Zn has widespread use (and, together with Fe, ranks among the
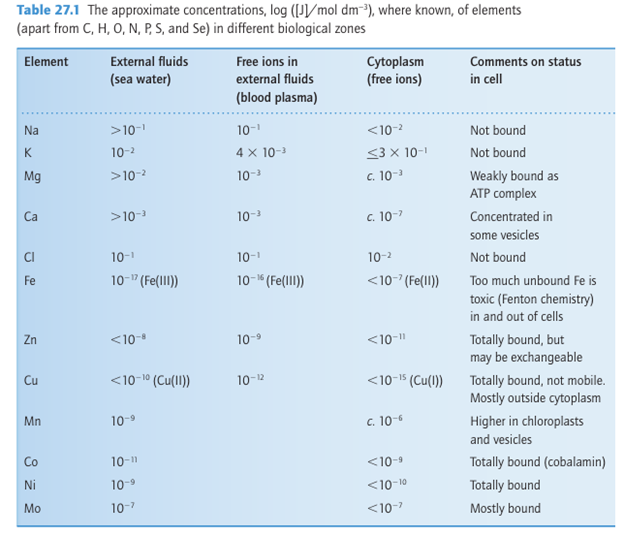
most abundant biological trace elements) whereas Co (a comparatively rare element) is essentially restricted to cobalamin. The early atmosphere (over 2.3 Ga ago1), being highly reducing, enabled Fe to be freely available as soluble Fe (II) salts, whereas Cu was trapped as insoluble sulfides (as was Zn). Indeed, Cu is not found in the archaea (which are be lieved to have evolved in pre-oxygenic times), including the hyperthermophiles, organisms that are able to survive at temperatures in excess of 100ºC. These organisms are found in deep sea hydrothermal vents and terrestrial hot springs and are good sources of enzymes that contain W, the heaviest element known to be essential to life. The finding that W, Co, and for the most part Ni are used only by more primitive life forms probably reflects their special role in the early stages of evolution.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


