
Calcium signalling proteins
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص733-734
الجزء والصفحة:
ص733-734
 2025-10-22
2025-10-22
 61
61
Calcium signalling proteins
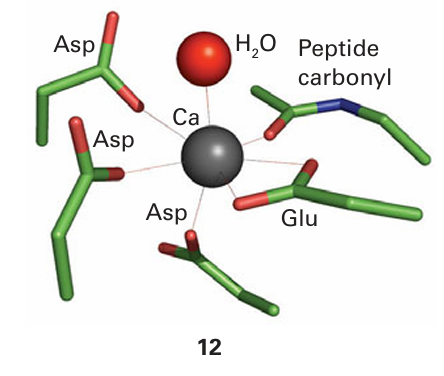
Key point: Calcium ions are suitable for signalling because they exhibit fast ligand exchange and a large, flexible coordination geometry. Calcium ions play a crucial role in higher organisms as an intracellular messenger, providing a remarkable demonstration of how organisms have exploited the otherwise rather limited chemistry of this element. Fluxes of Ca2+ trigger enzyme action in cells in response to receiving a hormonal or electrical signal from elsewhere in the organism. Calcium is particularly suited for signalling because it has fast ligand-exchange rates, intermediate binding constants, and a large, flexible coordination sphere. Calcium signalling proteins are small proteins that change their conformation depend ing on the binding of Ca2+ at one or more sites; they are thus examples of the metal ion activated proteins mentioned earlier. Every muscle movement we make is stimulated by Ca2+ binding to a protein known as troponin C. The best-studied Ca2-regulatory protein is calmodulin (17 kg mol–1, Fig. 27.8): its roles include activating protein kinases that catalyse phosphorylation of proteins and activating NO-synthase, a Fe-containing enzyme responsible for generating the intercellular signalling molecule nitric oxide. Calmodulin has four Ca2+-binding sites (one is shown as 12) with dissociation constants lying close to 10−6. The binding of Ca2+ to the four sites alters the protein conformation and it is then recognized by a target enzyme.5
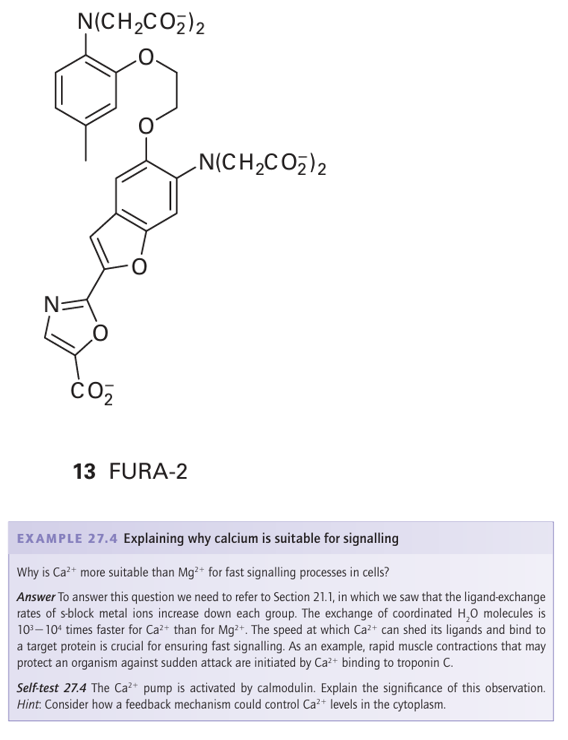
Calcium signalling requires special Ca2 pumps, which are large, membrane-spanning enzymes that pump Ca2 out of the cytoplasm, either out of the cell altogether or into Ca-storing organelles such as the endoplasmic reticulum or the mitochondria. As with Na /K-ATPases, the energy for Ca2 pumping comes from ATP hydrolysis. Hormones or electrical stimuli open specific channels (analogous to K channels) that release Ca2 into the cell. Because the level in the cytoplasm before the pulse is low, the influx easily raises the Ca2 concentration above that needed for Ca2-binding proteins such as calmodulin (or troponin C in muscle). The action can be short-lived, so that after a pulse of Ca2 the cell is quickly evacuated by the calcium pump. Although Ca2 is invisible to most spectroscopic methods, some Ca proteins, such as calmodulin or troponin C, are small enough to be studied by NMR. Because of their preference for large multicarboxylate ligands, the lanthanoid ions (Section 23.7) have been used as probes for Ca binding, exploiting their properties of paramagnetism (as chemical shift reagents in NMR spectroscopy) and fluorescence. Intracellular concentrations of Ca are monitored by using special fluorescent polycarboxylate ligands (13) that are introduced to the cell as their esters, which are hydrophobic and able to cross the membrane lipid barrier. Once in the cell, enzymes known as esterases hydrolyse the esters and release the ligands, which respond to changes in Ca2+ concentration in the range 107- –10-9 m.
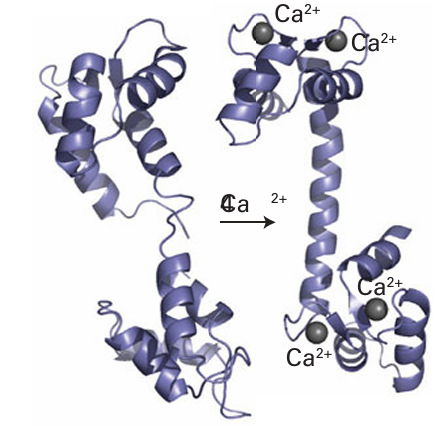
Figure 27.8 The binding of four Ca2+ to apocalmodulin causes a change in the protein conformation, converting it to a form that is recognized by many enzymes. The high proportion of α-helix is typical of proteins that are activated by metal-ion binding.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


