


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 23-6-2016
Date: 3-12-2015
Date: 17-9-2019
|
Drawing Resonance Forms
Look back at the resonance forms of the acetate ion and the acetone anion shown in the previous section. The pattern seen there is a common one that leads to a useful technique for drawing resonance forms. In general, any three atoms grouping with a p orbital on each atom has two resonance forms:
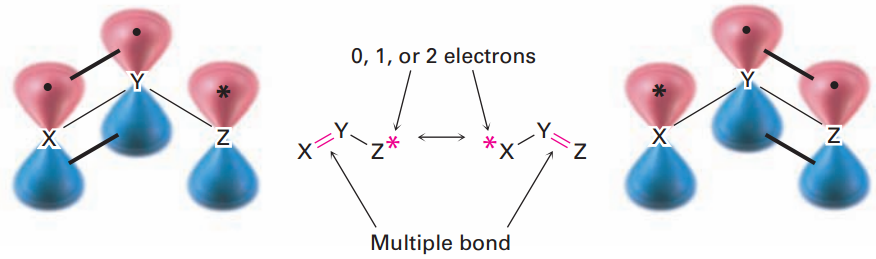
The atoms X, Y, and Z in the general structure might be C, N, O, P, S, or others, and the asterisk (*) might mean that the p orbital on atom Z is vacant, that it contains a single electron, or that it contains a lone pair of electrons.
The two resonance forms differ simply by an exchange in position of both the multiple bond and the asterisk from one end of the three-atom grouping to the other.
By learning to recognize such three-atom groupings within larger structures, resonance forms can be systematically generated. Look, for instance, at the anion produced when H+ is removed from 2,4-pentanedione by reaction with a base. How many resonance structures does the resultant anion have?
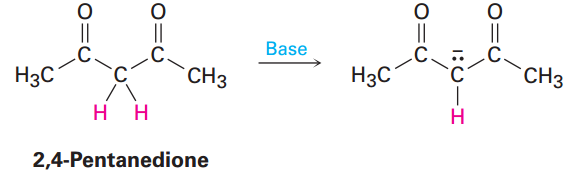
The 2,4-pentanedione anion has a lone pair of electrons and a formal negative charge on the central carbon atom, next to a C=O bond on the left. The O=C-C:- grouping is a typical one for which two resonance structures can be drawn.
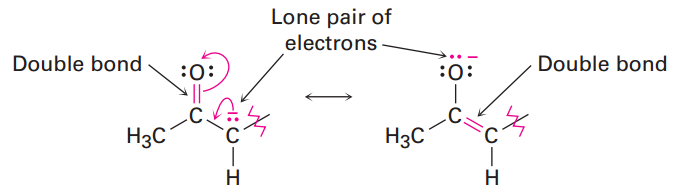
Just as there is a C=O bond to the left of the lone pair, there is a second C=O bond to the right. Thus, we can draw a total of three resonance structures for the 2,4-pentanedione anion.




|
|
|
|
تحذير من "عادة" خلال تنظيف اللسان.. خطيرة على القلب
|
|
|
|
|
|
|
دراسة علمية تحذر من علاقات حب "اصطناعية" ؟!
|
|
|
|
|
|
|
العتبة العباسية المقدسة تحذّر من خطورة الحرب الثقافية والأخلاقية التي تستهدف المجتمع الإسلاميّ
|
|
|