
(Case Study—Racemic and Non-Racemic Drugs (B
 المؤلف:
ADAM RENSLO
المؤلف:
ADAM RENSLO
 المصدر:
the organic chemistry of medicinal agent
المصدر:
the organic chemistry of medicinal agent
 الجزء والصفحة:
p 175
الجزء والصفحة:
p 175
 23-6-2016
23-6-2016
 2749
2749
Case Study—Racemic and Non-Racemic Drugs (B)
Ibuprofen, Ketoprofen, and Naproxen
Next we will consider the widely used nonsteroidal anti-inflammatory drugs (NSAIDs), which include ibuprofen, ketoprofen, and naproxen among others. The anti-inflammatory, analgesic, and antipyretic properties of NSAIDs result from their inhibition of the enzyme cyclooxygenase (COX). COX enzymes are involved in the biosynthesis of prostaglandins—biological small molecules that mediate a variety of processes such as inflammation and platelet aggregation. The various members of the “profen” class bear similar structural features, as one might expect given that these drugs target the same enzyme (Figure 1.1). Each of these molecules contains an aromatic ring system attached to the alpha carbon of propanoic acid, forming a single chirality center
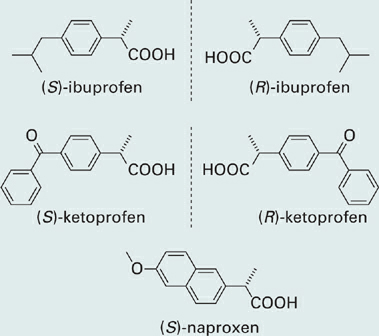
Figure 1.1 The R and S forms of the common nonsteroidal anti-inflammatory (NSAID) drugs ibuprofen and ketoprofen which have been developed both as racemic mixtures and as the pure S enantiomer. The NSAID (S)-naproxen was developed only in single-enantiomer form.
Of the two enantiomeric forms of these NSAIDs, only the S form is an effective inhibitor of COX enzymes. In the case of ibuprofen however, the inactive R enantiomer is converted in the body into the active S form by a metabolic process (fortuitously, the active S form is not converted to the inactive R form). This bioconversion would seem to mitigate any advantage of a single-enantiomer form of the drug—the R form is essentially a “pro-drug” that is converted in the body into the active drug species. Another factor to consider however is the possibility of adverse drug effects that might be associated with (R)-ibuprofen prior to its conversion to the active form. As a class, NSAIDs in fact do show a relatively high incidence of adverse effects, most commonly affecting the gastrointestinal tract and less commonly but more seriously involving the liver and kidneys. It also turns out that the bioconversion of (R)-ibuprofen to (S)-ibuprofen occurs at different rates in different individuals, a not inconsequential factor given that rapid drug action is desirable in an analgesic. For these reasons, (S)-ibuprofen was developed for use in single-enantiomer form and these products are now sold in some European countries.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


