


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 7-7-2020
Date: 24-6-2019
Date: 10-5-2020
|
The ionic radius is the radius of an atom forming ionic bond or an ion. The radius of each atom in an ionic bond will be different than that in a covalent bond. This is an important concept. The reason for the variability in radius is due to the fact that the atoms in an ionic bond are of greatly different size. One of the atoms is a cation, which is smaller in size, and the other atom is an anion which is a lot larger in size. So in order to account for this difference, one most get the total distance between the two nuclei and divide the distance according to atomic size. The bigger the atomic size, the larger radius it will have. This is depicted in Figure 1 as shown below where the cation is displayed on the left as X+, and clearly has a smaller radius than the anion, which is depicted as Y- on the right.
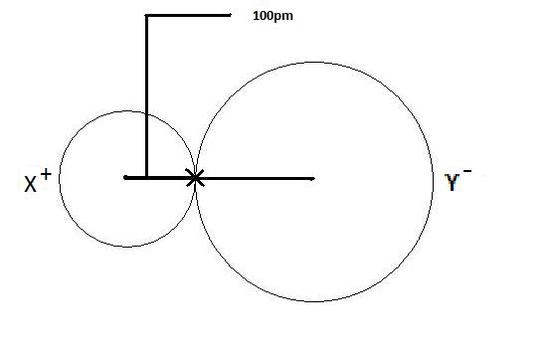
Figure 1: Ionic radii



|
|
|
|
دراسة تحدد أفضل 4 وجبات صحية.. وأخطرها
|
|
|
|
|
|
|
العتبة العباسية تستعدّ لتكريم عددٍ من الطالبات المرتديات للعباءة الزينبية في جامعات كركوك
|
|
|