

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
The Phenyl Group
المؤلف:
..................
المصدر:
LibreTexts Project
الجزء والصفحة:
.................
20-8-2019
1957
The Phenyl Group
As mentioned previously, the phenyl group (Ph-R, C6H5-R) can be formed by removing a hydrogen from benzene and attaching a substituent to where the hydrogen was removed. To this phenomenon, we can name compounds formed this way by applying this rule: (phenyl + substituent). For example, a chlorine attached in this manner would be named phenyl chloride, and a bromine attached in this manner would be named phenyl bromide. (See below diagram)
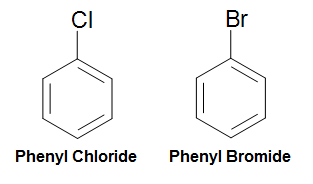
Figure 1. Naming of Phenyl Chloride and Phenyl Bromide
While compounds like these are usually named by simple benzene type naming (chlorobenzene and bromobenzene), the phenyl group naming is usually applied to benzene rings where a substituent with six or more carbons is attached, such as in the diagram below.

Figure 2. Diagram of 2-phenyloctane.
Although the diagram above might be a little daunting to understand at first, it is not as difficult as it seems after careful analysis of the structure is made. By looking for the longest chain in the compound, it should be clear that the longest chain is eight (8) carbons long (octane, as shown in green) and that a benzene ring is attached to the second position of this longest chain (labeled in red). As this rule suggests that the benzene ring will act as a function group (a substituent) whenever a substituent of more than six (6) carbons is attached to it, the name "benzene" is changed to phenyl and is used the same way as any other substituents, such as methyl, ethyl, or bromo. Putting it all together, the name can be derived as: 2-phenyloctane (phenyl is attached at the second position of the longest carbon chain, octane).
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















