


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 12-5-2016
Date: 16-8-2019
Date: 14-8-2019
|
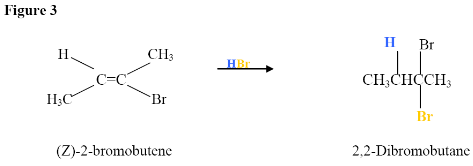
Here, the electrophilic addition proceeds with the same steps used to achieve the product in Addition of a HX to an Internal Alkyne. The π
electrons attacked the hydrogen, adding it to the carbon on the left (shown in blue). Why was hydrogen added to the carbon on left and the one on the right bonded to the Bromine?
Now, you will have your carbocation intermediate, which is followed by the attack of the Bromine to the carbon on the right resulting in a haloalkane product.
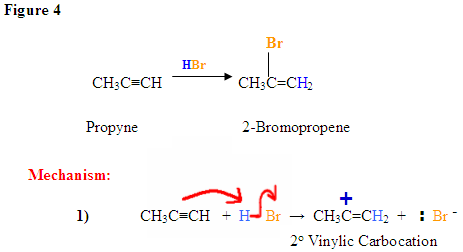
The π electrons are attacking the hydrogen, depicted by the electron pushing arrows and the Bromine gains a negative charge. The carbocation intermediate forms a positive charge on the left carbon after the hydrogen was added to the carbon with the most hydrogen substituents.

The Bromine, which has a negative charge, attacks the positively charged carbocation forming the final product with the nucleophile on the more substituted carbon.



|
|
|
|
دراسة تحدد أفضل 4 وجبات صحية.. وأخطرها
|
|
|
|
|
|
|
جامعة الكفيل تحتفي بذكرى ولادة الإمام محمد الجواد (عليه السلام)
|
|
|