


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 19-12-2021
Date: 3-9-2021
Date: 1-10-2021
|
Structure of collagen
Unlike most globular proteins that are folded into compact structures, collagen, a fibrous protein, has an elongated, triple-helical structure that is stabilized by interchain hydrogen bonds.
1. Amino acid sequence: Collagen is rich in proline and glycine, both of which are important in the formation of the triple-stranded helix. Proline facilitates the formation of the helical conformation of each α chain because its ring structure causes “kinks” in the peptide chain. [Note: The presence of proline dictates that the helical conformation of the α chain cannot be an α helix ] .Glycine, the smallest amino acid, is
found in every third position of the polypeptide chain. It fits into the restricted spaces where the three chains of the helix come together. The glycine residues are part of a repeating sequence, −Gly–X–Y–, where X is frequently proline, and Y is often hydroxyproline (but can be hydroxylysine, Fig. 1). Thus, most of the α chain can be regarded as a polytripeptide whose sequence can be represented as (−Gly–Pro–Hyp–).
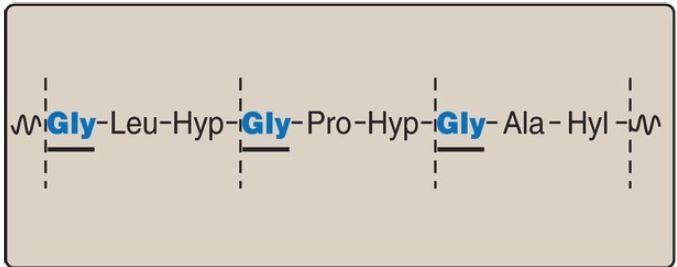
Figure 1: Amino acid sequence of a portion of the α1 chain of collagen. Hyp =hydroxyproline; Hyl = hydroxylysine.
2. Hydroxyproline and hydroxylysine: Collagen contains hydroxyproline and hydroxylysine, which are nonstandard amino acids not present in most other proteins. They result from the hydroxylation of some of the proline and lysine residues after their incorporation into polypeptide chains (Fig. 2). Therefore, the hydroxylation is a posttranslational modification . [Note: Generation of hydroxyproline maximizes formation of interchain hydrogen bonds that stabilize the triple-helical structure.]
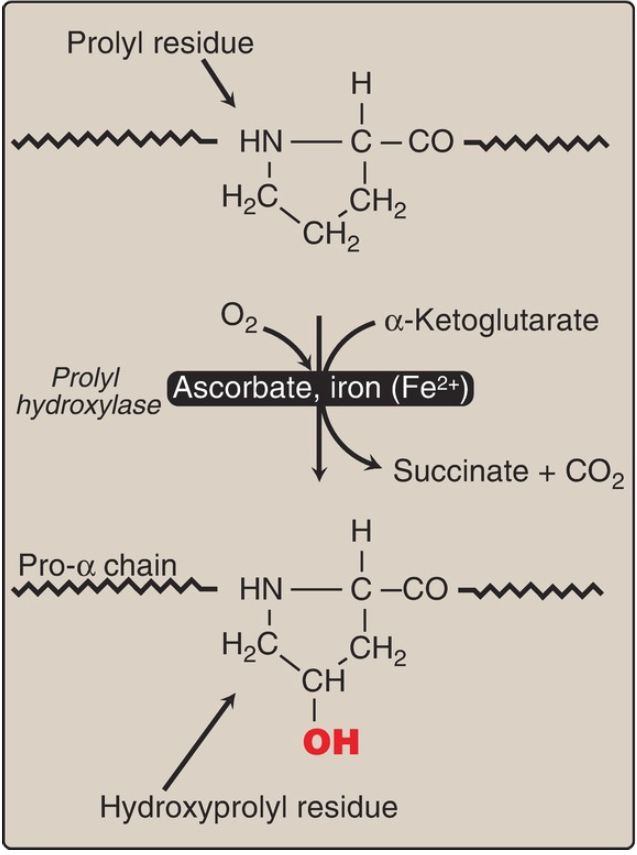
Figure 2: Hydroxylation of proline residues in pro-α chains of collagen by prolyl hydroxylase. [Note: Fe2+ (hydroxylase cofactor) is protected from oxidation to Fe3+ by ascorbate (vitamin C).]
3. Glycosylation: The hydroxyl group of the hydroxylysine residues of collagen may be enzymatically glycosylated. Most commonly, glucose and galactose are sequentially attached to the polypeptide chain prior to triple-helix formation (Fig. 3).
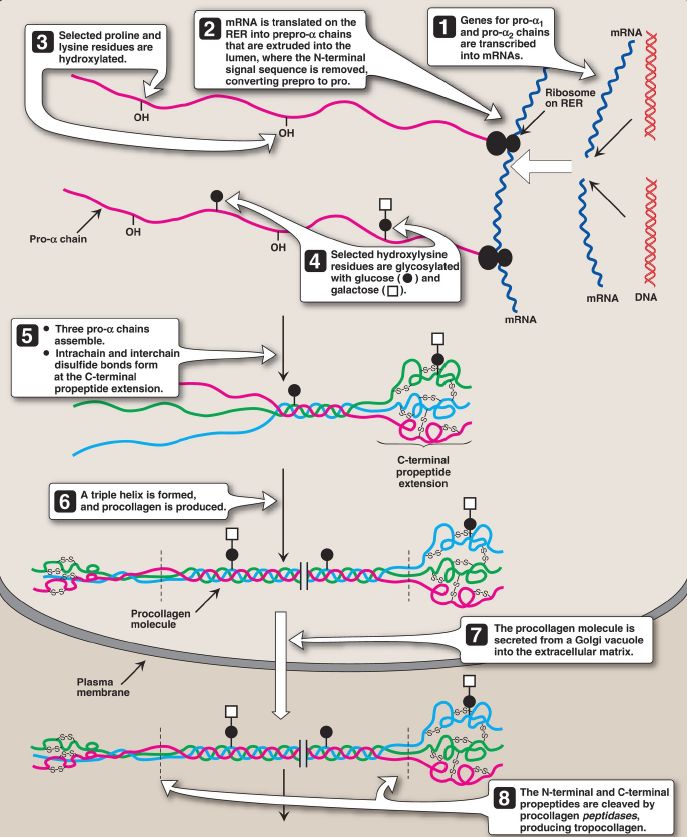
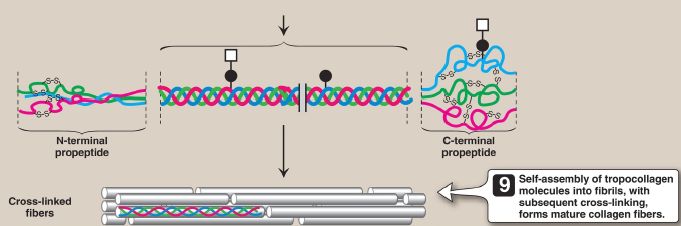
Figure 3: Synthesis of collagen. RER = rough endoplasmic reticulum; mRNA =messenger RNA.



|
|
|
|
تحذير من "عادة" خلال تنظيف اللسان.. خطيرة على القلب
|
|
|
|
|
|
|
دراسة علمية تحذر من علاقات حب "اصطناعية" ؟!
|
|
|
|
|
|
|
العتبة العباسية المقدسة تحذّر من خطورة الحرب الثقافية والأخلاقية التي تستهدف المجتمع الإسلاميّ
|
|
|