
آخر المواضيع المضافة


 الفيزياء الكلاسيكية
الفيزياء الكلاسيكية
 الكهربائية والمغناطيسية
الكهربائية والمغناطيسية
 علم البصريات
علم البصريات
 الفيزياء الحديثة
الفيزياء الحديثة
 النظرية النسبية
النظرية النسبية
 الفيزياء النووية
الفيزياء النووية
 فيزياء الحالة الصلبة
فيزياء الحالة الصلبة
 الليزر
الليزر
 علم الفلك
علم الفلك
 المجموعة الشمسية
المجموعة الشمسية
 الطاقة البديلة
الطاقة البديلة
 الفيزياء والعلوم الأخرى
الفيزياء والعلوم الأخرى
 مواضيع عامة في الفيزياء
مواضيع عامة في الفيزياء|
Read More
Date: 6-9-2016
Date: 25-7-2016
Date: 4-9-2016
|
Diesel Cycle
Calculate the efficiency of the Diesel cycle, consisting of two adiabats, 1→2 and 3→4, one isobar 2→3, and one constant-volume process 4→1 (see Figure 1.1). Assume CV and CP are constant.
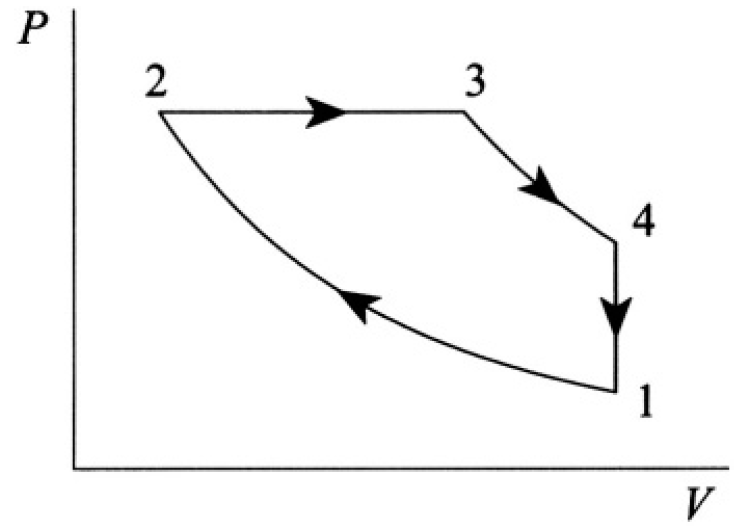
Figure 1.1
SOLUTION
We calculate the efficiency η = W/Q. The work W in
the cycle (see Figure 1.2) is
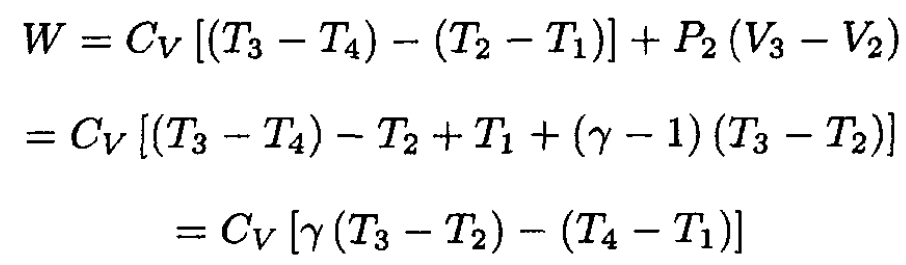 (1)
(1)
where we have again used the ideal gas law PV = nRT and nR = CP - CV. The heat Q absorbed by the gas during 2 → 3 is
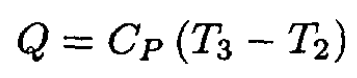 (2)
(2)
The efficiency is
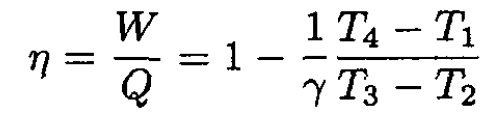 (3)
(3)
Using the equation for the adiabats gives
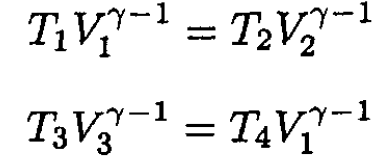 (4)
(4)
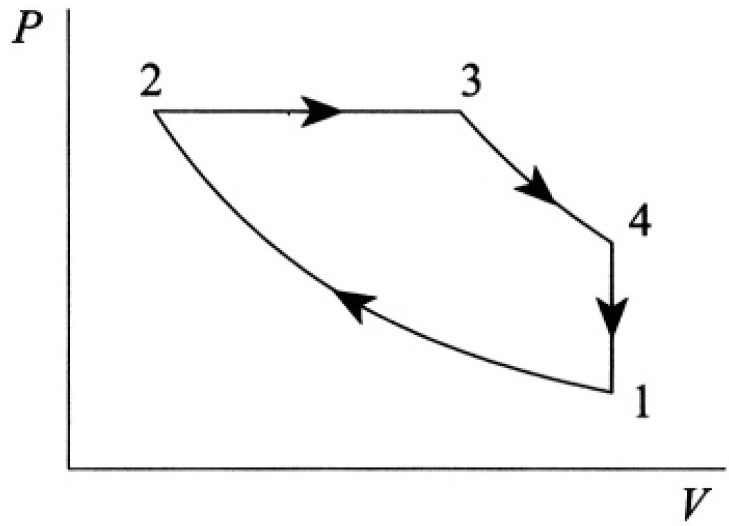
Figure 1.2
The ideal gas law gives
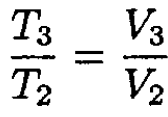 (5)
(5)
Substituting (4) and (5) into (3) gives
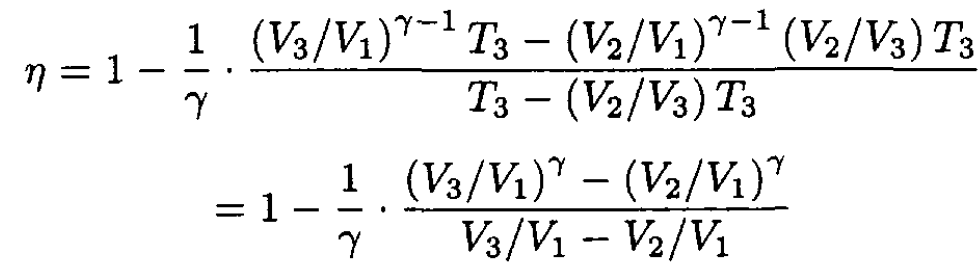 (6)
(6)



|
|
|
|
دراسة تكشف "مفاجأة" غير سارة تتعلق ببدائل السكر
|
|
|
|
|
|
|
أدوات لا تتركها أبدًا في سيارتك خلال الصيف!
|
|
|
|
|
|
|
العتبة العباسية المقدسة تؤكد الحاجة لفنّ الخطابة في مواجهة تأثيرات الخطابات الإعلامية المعاصرة
|
|
|