
آخر المواضيع المضافة


 الفيزياء الكلاسيكية
الفيزياء الكلاسيكية
 الكهربائية والمغناطيسية
الكهربائية والمغناطيسية
 علم البصريات
علم البصريات
 الفيزياء الحديثة
الفيزياء الحديثة
 النظرية النسبية
النظرية النسبية
 الفيزياء النووية
الفيزياء النووية
 فيزياء الحالة الصلبة
فيزياء الحالة الصلبة
 الليزر
الليزر
 علم الفلك
علم الفلك
 المجموعة الشمسية
المجموعة الشمسية
 الطاقة البديلة
الطاقة البديلة
 الفيزياء والعلوم الأخرى
الفيزياء والعلوم الأخرى
 مواضيع عامة في الفيزياء
مواضيع عامة في الفيزياء|
Read More
Date: 4-9-2016
Date: 11-8-2016
Date: 13-7-2016
|
Isochoric Cooling and Isobaric Expansion
An ideal gas of total mass m and molecular weight μ is isochorically (at constant volume) cooled to a pressure times smaller than the initial pressure P1. The gas is then expanded at constant pressure so that in the final state the temperature T2 coincides with the initial temperature T1. Calculate the work done by the gas.
SOLUTION
The process diagram is shown in Figure 1.1. The work W done by the gas occurs only during the 2→3 leg since there is no work done during the 1→2 leg. The work is given by
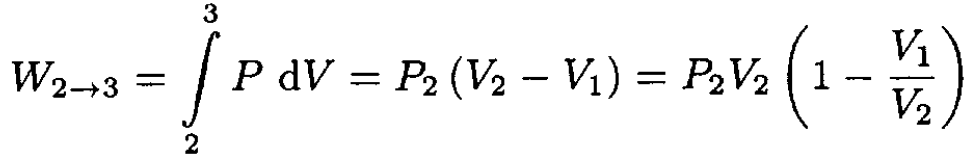 (1)
(1)
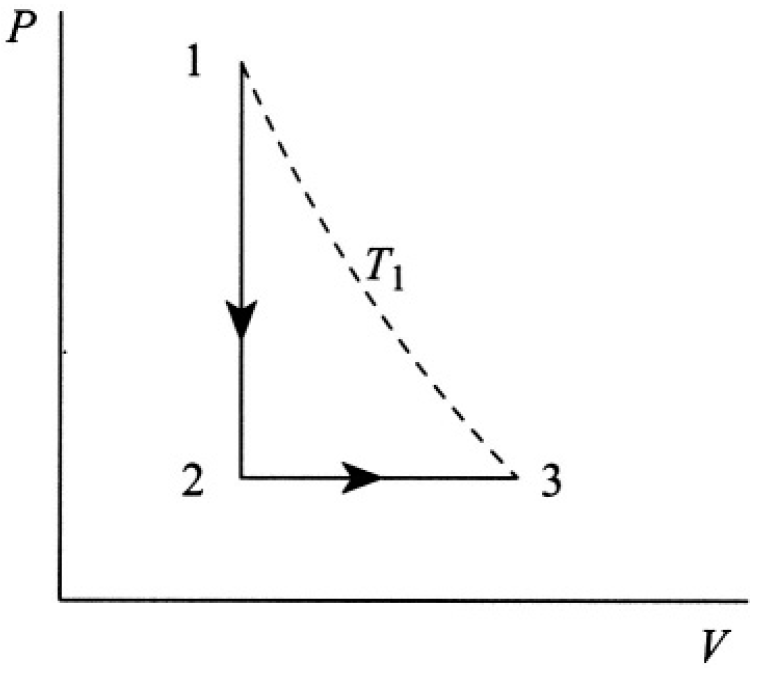
Figure 1.1
Using the ideal gas law, we may relate V1 and V2:
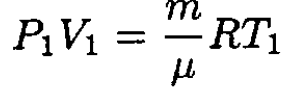 (2)
(2)
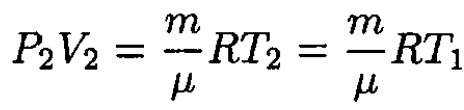
since the initial and final temperatures are the same. Substituting into (1) we find
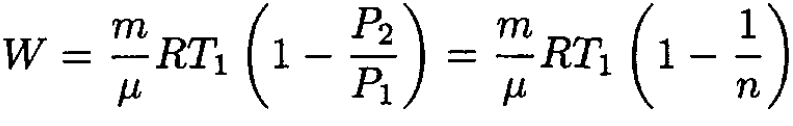 (3)
(3)



|
|
|
|
دراسة تكشف "مفاجأة" غير سارة تتعلق ببدائل السكر
|
|
|
|
|
|
|
أدوات لا تتركها أبدًا في سيارتك خلال الصيف!
|
|
|
|
|
|
|
العتبة العباسية المقدسة تؤكد الحاجة لفنّ الخطابة في مواجهة تأثيرات الخطابات الإعلامية المعاصرة
|
|
|