


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 24-6-2017
Date: 24-1-2017
Date: 17-2-2019
|
Metals
If you look carefully at Figure 1.1, you can see a stair-stepped line starting at boron (B), atomic number 5, and going all the way down to polonium (Po), atomic number 84.

Except for germanium (Ge) and antimony (Sb), all the elements to the left of that line can be classified as metals. Figure 1.2 shows the metals.
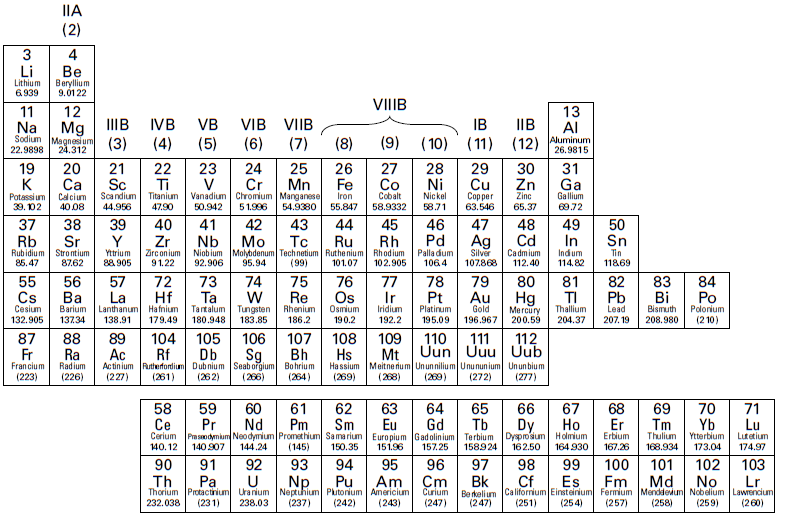
These metals have properties that you normally associate with the metals you encounter in everyday life. They’re solid at room temperature (with the exception of mercury, Hg, a liquid), shiny, good conductors of electricity and heat, ductile (they can be drawn into thin wires), and malleable (they can be easily hammered into very thin sheets). All these metals tend to lose electrons easily. As you can see, the vast majority of the elements on the periodic table are classified as metals.



|
|
|
|
لشعر لامع وكثيف وصحي.. وصفة تكشف "سرا آسيويا" قديما
|
|
|
|
|
|
|
كيفية الحفاظ على فرامل السيارة لضمان الأمان المثالي
|
|
|
|
|
|
|
شعبة مدارس الكفيل: مخيَّم بنات العقيدة يعزِّز القيم الدينية وينمِّي مهارات اتخاذ القرار لدى المتطوِّعات
|
|
|